Oxygen-induced abnormal photoelectric behavior of a MoO3/graphene heterocomposite†
Abstract
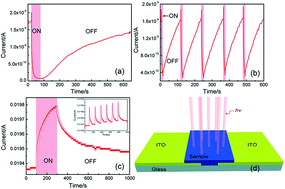
A MoO3 nanoflakes/graphene heterocomposite synthesized by a water bath method showed unusual photoelectric behavior. Its resistance increased under visible light irradiation in air. The behavior arises from the physical contact between the two materials, which leads to hole doping in graphene. Thus, the increase of electrons in the MoO3 nanoflakes induced by desorption of oxygen molecules from its surface under visible light was probably the main reason for the change in resistance. This was confirmed by the resistance of the heterocomposite in a vacuum being 102 times larger than that in air, and by the photoelectric measurements under Ar. The MoO3 nanoflakes/graphene heterocomposite exhibits great potential for use in oxygen gas sensing.

 Please wait while we load your content...
Please wait while we load your content...