Retracted Article: Multi-walled carbon nanotubes decorated with palladium nanoparticles as a novel platform for electrocatalytic sensing applications
Abstract
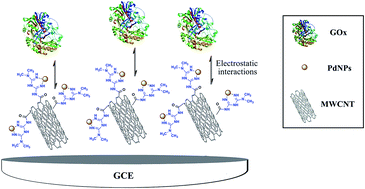
A simple method is described for decorating multi-walled carbon nanotubes (MWCNTs) with palladium nanoparticles (PdNPs). MWCNTs were functionalized via a C–C covalent bond with metformin (Met/fMWCNT). The subsequent bonding of the imine groups with palladium offered strong adhesion of PdNPs on functionalized MWCNT surface (Pd@Met/fMWCNT). The structure and morphology of the resulting MWCNT-based nanocomposite were characterized by transmission electron microscopy, X-ray diffraction and FT-IR. Pd@Met/fMWCNT nanocomposite was used to immobilize glucose oxidase (GOx) on glassy carbon electrode. Detailed electrochemical analysis was performed and it was found that Pd@Met/fMWCNT nanocomposite significantly improved direct electron transfer between GOx and glassy carbon electrode, leading to fabrication of a potential biosensor offering very sensitive detection of glucose. The surface coverage of GOx and the electron transfer rate constant (ks) were calculated to be 4.17 × 10−10 mol cm−2 and 3.24 s−1, respectively. The apparent Michaelis–Menten constant of the immobilized GOx was 0.48 mM, implying a strong catalytic activity and a remarkable affinity of the immobilized GOx for glucose. The linear detection range of glucose was 4.0–1500 μM, with a detection limit of 1.4 ± 0.04 μM.
- This article is part of the themed collection: Nanoscience and nanotechnology in electrochemistry

 Please wait while we load your content...
Please wait while we load your content...