Natural, biodegradable and flexible egg shell membranes as separators for lithium-ion batteries
Abstract
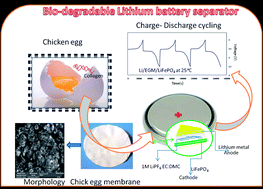
Flexible egg shell membranes (ESM) were obtained from chicken eggs after treatment with hydrochloric acid. The ESMs were subjected to scanning electron microscopy (SEM) and thermogravimetric (TG) and wettability analyses. The morphological studies revealed that the membranes possessed uniform porosity and they were of micron size. The ESM was also found to be thermally stable above 230 °C. The electrochemical properties, such as ionic conductivity, lithium transport number (Lit+) and compatibility of the membrane upon activation in 1 M LiPF6 in EC : DMC (1 : 1 v/v), were analyzed. Finally, a 2032-type coin cell composed of Li/ESM/LiFePO4 was assembled and its cycling profile was also analyzed at different C-rates.

 Please wait while we load your content...
Please wait while we load your content...