The control of crystallographic texture in the use of magnesium as a resorbable biomaterial
Sumit Bahl,
Satyam Suwas and
Kaushik Chatterjee*
Department of Materials Engineering, Indian Institute of Science, Bangalore 560012, India. E-mail: kchatterjee@materials.iisc.ernet.in; Tel: +91-80-22933408
First published on 10th October 2014
Abstract
Magnesium and its alloys are an emerging class of resorbable materials for orthopedic and cardiovascular applications. The typical strategy underlying the development of these materials involves the control of material processing routes and the addition of alloying elements. Crystallographic texture is known to control bulk mechanical as well as surface properties. However, its role in determining the properties of magnesium for implant materials has not been well studied. In this work, an extruded rod of pure magnesium was cut in multiple directions to generate samples with different textures. It was found that texture significantly affected the strength and ductility of magnesium. Corrosion rates in Hank's solution decreased with the increased presence of low energy basal planes at the surface. In vitro cell studies revealed that changes in texture did not induce cytotoxicity. Thus, the control of texture in magnesium based implants could be used to tailor the mechanical properties and the resorption rates without compromising cytocompatibility. This study elucidates the importance of texture in the use of magnesium as a resorbable biomaterial.
1. Introduction
There is considerable interest in the development of resorbable biomaterials, as they can be eliminated from the body after the intended application without the need for a secondary surgical procedure. These materials offer promising new alternatives for implants used in orthopedic and cardiovascular applications. Fracture fixation requires metallic plates, screws and rods to hold the bone parts together until the bone is regenerated. Currently, these implants are made of non-resorbable materials such as titanium and its alloys, stainless steels and Co–Cr alloys.1 In many cases, a second surgery is performed to remove these implants after the fracture is healed. This increases costs, morbidity at the implant site, risk of infection and pain to the patient.2 Another application of resorbable implants is coronary stents used in the opening of blocked arteries, which supply blood to the heart.3,4 It is believed that stents are not required after a certain time as the arterial wall remodels and attains equilibrium due to the stresses applied by the stent. The presence of stents for prolonged periods leads to complications such as in-stent restenosis and thrombosis, and requires long term use of anti-thrombotic drugs which increases the risk of hemorrhage.3,4 Thus, it is important to develop materials that are resorbed naturally and excreted out of the body after their desired function is accomplished.Magnesium and its alloys are an important class of resorbable metallic biomaterials for orthopedic and cardiovascular applications.1,3–6 Magnesium is favored over ceramic and polymeric biomaterials as it offers a good combination of high strength, fracture toughness and elastic modulus, which closely matches that of bone. Magnesium is a part of human metabolism and is found mainly in bone tissues. Therefore, the controlled release of magnesium ions from degrading implants may not be toxic and the ions can be easily excreted out of the body. In fact, the presence of magnesium ions is reported to have a stimulatory effect on regeneration of bone tissue.7
The high corrosion rate of magnesium compared to other metals makes it suitable for resorbable applications. Although this is an advantage, the current magnesium alloys corrode in physiological conditions before the bone is fully regenerated. Implants lose their mechanical strength due to rapid corrosion, leading to their failure.1,8 Therefore, one of the major challenges in the development of magnesium alloys for resorbable biomedical applications is the control of the corrosion rate. The commonly used methods to control the corrosion rate include alloying, processing and surface treatments. Some of the widely studied alloying elements include Ca, Al, Mn, Zn, Zr, and Si.9–12 It has been shown that magnesium alloys processed to obtain fine grain size show an enhanced corrosion resistance.13 Various surface modification techniques such as micro-arc oxidation, alkali treatment, chemical conversion coatings, and sol–gel coatings have been applied to improve the corrosion resistance of magnesium.6,14,15
Apart from corrosion, the other important requirement for an implant is mechanical properties such as strength, ductility and elastic modulus. Incidentally, the corrosion as well as the mechanical properties are strongly dependent on crystallographic texture. Crystallographic texture in a polycrystalline material is defined as an occurrence of preferred orientation of grains within the material. It is widely used in numerous engineering applications to tune material properties. The texture of magnesium alloys is known to significantly affect the corrosion resistance.16,17 Given the anisotropy associated with its hexagonal crystal structure(Fig. 1(a)),18,19 mechanical properties such as strength, ductility, and elastic modulus are especially sensitive to the texture of magnesium. Control of texture to tailor properties has been successfully employed in many industries, especially for automobile and aerospace applications. Although the effect of texture on structural properties and corrosion behavior is well established, its effects, if any, on the biological response to a material are poorly understood. A few recent investigations20–22 have reported effect of texture on the cellular response to titanium and its alloys. However, the use of texture as a tool to tune properties to optimize the performance of materials is yet to be fully exploited for biomedical applications and there are no known reports on resorbable biomaterials such as magnesium.
The aim of this study is to investigate the effect of texture on the mechanical properties, corrosion behavior and biocompatibility of commercially pure magnesium for potential use in orthopedic applications. An extruded rod of pure magnesium was cut in three directions (Fig. 1(b)). Thus, the samples had similar grain size distribution but different textures. The mechanical properties and corrosion resistance for the different textures were systematically characterized. Biocompatibility was evaluated by studying the effect of leached magnesium ions on mouse osteoblasts as a model for their use in orthopedic applications.
2. Materials and methods
2.1 Material and processing
Indigenously melted and casted magnesium was used for this work (composition in wt % – Mg: 99.93, Al: 0.0032, Mn: 0.0128, Cu: 0.0005, Fe: 0.0017, Si: 0.0228, Ni: 0.0003). The cast billets were pre-heated at 400 °C for 2 h followed by extrusion at a speed of 10 mm s−1. The cross-section after extrusion was 15 mm × 15 mm, equivalent to an extrusion ratio of 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The extruded rod was cut in three directions, as shown schematically in Fig. 1, such that the surface normal was parallel, perpendicular and at 45° with respect to the extrusion axis. These samples will be hereafter referred to as transverse, longitudinal and 45° respectively (Fig. 1(b)).
1. The extruded rod was cut in three directions, as shown schematically in Fig. 1, such that the surface normal was parallel, perpendicular and at 45° with respect to the extrusion axis. These samples will be hereafter referred to as transverse, longitudinal and 45° respectively (Fig. 1(b)).
2.2 Microstructure and texture
Characterization of microstructure and texture was carried out using the scanning electron microscope (SEM) based electron back scattered diffraction (EBSD) technique. EBSD was carried out on an ESEM-Quanta with an EBSD set-up provided by TexSEM laboratories (Draper). Analysis of data was performed using TSL-OIM software version 5.2. Crystallographic texture was analyzed along three directions, namely, perpendicular, at 45° and parallel to the extrusion axis, for EBSD data. The samples were polished with standard metallographic techniques followed by electro-polishing using an electrolyte consisting of orthophosphoric acid and ethanol in a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ratio (v/v). The electro-polishing was performed at 3.0 V for 30 s and 1.5 V for 120 s to produce a strain free surface for EBSD examination. EBSD data was recorded with a step size of 2 μm. A large number of grains were covered to account for statistics necessary for texture analysis.
5 ratio (v/v). The electro-polishing was performed at 3.0 V for 30 s and 1.5 V for 120 s to produce a strain free surface for EBSD examination. EBSD data was recorded with a step size of 2 μm. A large number of grains were covered to account for statistics necessary for texture analysis.
2.3 Corrosion
Potentio-dynamic polarization was used to evaluate the corrosion properties of the samples in Hank's solution using a potentiostat (Gill AC). The composition of the solution used was as follows: 8.00 g L−1 NaCl, 1.00 g L−1 glucose, 0.40 g L−1 KCl, 0.35 g L−1 NaHCO3, 0.14 g L−1 CaCl2, 0.10 g L−1 MgCl2, 0.06 g L−1 Na2HPO4, 0.06 g L−1 KH2PO4, and 0.06 g L−1 MgSO4. The samples were prepared by grinding with SiC papers up to P3000 grit followed by final polishing using 0.25 μm diamond paste to obtain a mirror finish. Samples were ultrasonically cleaned in methanol and dried before beginning the test. The polarization test was conducted with Pt as counter electrode, saturated calomel as reference electrode and sample as working electrode. The sample was immersed in the corrosion media for 30 min to stabilize the potential (rest potential) before starting the polarization. The polarization was performed at a scan rate of 12 mV min−1 from −150 mV to + 1500 mV relative to the rest potential. The cathodic polarization curve of the Tafel plots was used to calculate the corrosion potential (Ecorr) and corrosion current density (Icorr).2.4 Mechanical testing
The effect of texture on mechanical properties was studied by compression test. The samples were tested along three directions, namely, perpendicular, at 45° and parallel to the extrusion axis. The tests were conducted at a strain rate of 10−3 until fracture. Adequate lubrication was provided at the contact surfaces between the sample and the grip to eliminate the effect of friction.2.5 Biocompatibility
The effect of texture on biocompatibility was studied using the MC3T3-E1 sub-clone 4 (ATCC) mouse osteoblast cell line. This is a well-established osteoblast model.23 An indirect method was used to evaluate biocompatibility. The cells were cultured in alpha-Minimum Essential Medium (α-MEM) with 10% (v/v) fetal bovine serum (FBS, Gibco, Life Technologies). Antibiotic penicillin–streptomycin (Sigma) was also added at 1% (v/v) concentration. Cells were passaged with trypsin–EDTA and subsequently sub-cultured. Cells of passage 3 were used for all the reported studies. The conditioned medium for the indirect assay was prepared by immersing Mg samples in growth medium. The ratio of sample area to medium volume was kept constant at 15 μl mm−2 for all the immersed samples. Samples were immersed in growth medium and incubated for 24 h at 37 °C and 5% CO2. The conditioned medium was centrifuged at 5000 rpm for 30 min to remove corrosion debris. Three replicates per sample were immersed and 200 μl of cell suspension containing 5 × 103 cells was added to each well of a 96-well plate. The growth medium was replaced with 200 μl of conditioned medium after 24 h of seeding cells.An MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide; thiazolyl blue) assay (Sigma) was used to measure cell viability at 1 day and 3 days after adding the conditioned medium. A stock solution of 5 mg ml−1 was prepared by dissolving MTT powder in sterile phosphate buffered saline (PBS). A working solution was prepared by diluting the stock solution to 1 mg ml−1 in growth medium. 100 μl of working solution was added to each well and incubated at 37 °C and 5% CO2 for 2 h. The dye is reduced to purple colored formazan crystals by dehydrogenase enzyme produced in the mitochondria of viable cells. The crystals were dissolved by adding 150 μl of DMSO to each well and incubating for 15 min at 37 °C. The dye concentration was quantified by measuring absorbance at 570 nm using a micro-well plate reader (Biotek). All data is presented as mean ± S.D. for n = 3.
Mg2+ concentration in conditioned medium was measured using an atomic absorption spectrometer (AAS, Thermo Scientific). A standard curve was drawn using samples containing known concentrations of Mg2+. The conditioned medium was diluted 75 times in ultrapure water (Sartorius) before measurement to bring the concentration within the range of the standard curve. The pH of the conditioned medium was measured using a standard pH meter.
The effects of Mg2+ ion concentration and pH on osteoblast viability were studied. Mg2+ concentration was varied in growth medium by exogenous addition of known quantities of MgCl2. The concentration of Mg2+ in the medium was varied from 102 μg ml−1 to 104 μg ml−1. Growth medium containing an endogenous Mg2+ concentration of 20 μg ml−1 was used as the control. The pH of the medium was varied by adding NaOH solution. The final pH values of the media prepared were adjusted to 8.0, 9.0, 10.0, 11.0 and 12.0. Growth medium of pH 7.4 was used as a control. 200 μl of cell suspension containing 5 × 103 cells was added to each well. The growth medium with controlled Mg2+ concentration and pH was added after 24 h of seeding cells. MTT was used to measure osteoblast viability at 1 day and 3 days after replacing growth medium following the procedures as described above. All data is presented as mean ± S.D for n = 3.
Statistical analyses were performed using one-way ANOVA (analysis of variance) with Tukey's test. Differences were considered significant for p < 0.05.
3. Results and discussion
3.1 Microstructure and texture
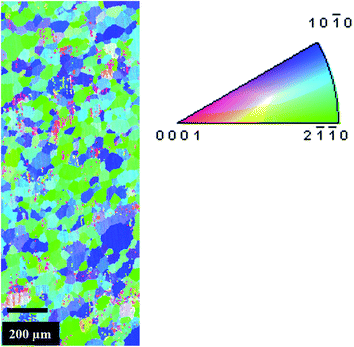
Fig. 2 presents an EBSD generated oriented imaging micrograph on the transverse plane. The microstructure is equi-axed with an average grain size of 20 μm. The overall orientation is depicted in Fig. 3 through inverse pole figure (IPF) plots. The colored lines in Fig. 3 are iso-intensity contour lines. Each color is associated with a value ‘N’, which is the normalized intensity with respect to a randomly textured sample. The normalized intensity of a randomly textured sample at every point in the pole figure will be ‘1’, indicating that all orientations are present with equal probability. The values are assigned in the legend in the same figure. A value of N > 1 indicates an occurrence of preferred orientation (texture) in the sample. The IPF plots for the transverse, longitudinal and 45° samples indicate that for the transverse sample the surface normal (extrusion axis) lies perpendicular to the prismatic planes (10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) and (11
0) and (11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0) of hexagonal crystal. This is schematically depicted in Fig. 1(b), where the extrusion axis lies perpendicular to the prismatic planes. In other words, the prismatic planes are parallel to the surface of the transverse sample. The IPF for the longitudinal sample shows that the surface normal lies perpendicular to the (0001) basal planes. This implies that basal planes are parallel to the surface of the longitudinal samples. The orientation of the 45° sample lies in between the prismatic and basal orientations as seen in the corresponding IPF (Fig. 3).
0) of hexagonal crystal. This is schematically depicted in Fig. 1(b), where the extrusion axis lies perpendicular to the prismatic planes. In other words, the prismatic planes are parallel to the surface of the transverse sample. The IPF for the longitudinal sample shows that the surface normal lies perpendicular to the (0001) basal planes. This implies that basal planes are parallel to the surface of the longitudinal samples. The orientation of the 45° sample lies in between the prismatic and basal orientations as seen in the corresponding IPF (Fig. 3).
Table 1 compiles the calculated volume fractions of basal and prismatic fibers for the three samples. The transverse sample has the highest volume fraction of prismatic fiber and lowest fraction of basal fiber, as the prismatic planes are parallel to its surface. The longitudinal sample has the highest fraction of basal fiber and lowest fraction of prismatic fiber as the basal planes are parallel to its surface. The 45° sample has lower volume fractions of both prismatic and basal fiber as its lies in between the prismatic and basal orientations. To summarize the texture of extruded Mg, the prismatic planes are perpendicular and the basal planes are parallel to the extrusion axis as is shown schematically in Fig. 1(b).
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) and (11
0) and (11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0) prismatic fibers for transverse, longitudinal and 45° samples
0) prismatic fibers for transverse, longitudinal and 45° samples
| Sample | Volume fraction of fibers | ||
|---|---|---|---|
| (0001) | (10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) 0) |
(11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0) 0) |
|
| Transverse | 0.1 | 18.9 | 19.5 |
| Longitudinal | 8.2 | 1.0 | 1.0 |
| 45° | 0.1 | 5.6 | 6.7 |
3.2 Mechanical properties
Fig. 4 presents the stress–strain plots of the compression test for the different textures. Table 2 summarizes the strength and ductility determined from the compression curves. The transverse sample exhibited the highest strength. The longitudinal and 45° samples had similar strengths but they were lower than that of the transverse sample. The ductility was lower for the longitudinal sample than for the transverse and 45° samples. Note that texture is the only variable among the three samples and therefore, underlies the differences in mechanical properties. Deformation mechanisms operating in Mg are dependent on the texture. Basal slip and {10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 2} tensile twinning are the dominant deformation modes active in Mg. The critical resolved shear stress (CRSS) for basal slip in Mg as measured by single crystal experiments is nearly 0.75 MPa, whereas the CRSS for prismatic slip is nearly 40 MPa.24 The CRSS for tensile twinning is reported to be 10 MPa.25 The other deformation modes observed in Mg are prismatic/pyramidal slip and {10
2} tensile twinning are the dominant deformation modes active in Mg. The critical resolved shear stress (CRSS) for basal slip in Mg as measured by single crystal experiments is nearly 0.75 MPa, whereas the CRSS for prismatic slip is nearly 40 MPa.24 The CRSS for tensile twinning is reported to be 10 MPa.25 The other deformation modes observed in Mg are prismatic/pyramidal slip and {10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 1}/{10
1}/{10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 3} compressive twinning. Prismatic and pyramidal slip are observed only above 300 °C in pure magnesium25 although micro-compression experiments of Mg single crystals loaded along the c-axis have shown pyramidal slip activity.26 The room temperature (RT) CRSS for compression twining is estimated to be between 100–150 MPa.25 In light of these data, basal slip and tensile twinning seem to be the active deformation modes in RT compression of Mg.
3} compressive twinning. Prismatic and pyramidal slip are observed only above 300 °C in pure magnesium25 although micro-compression experiments of Mg single crystals loaded along the c-axis have shown pyramidal slip activity.26 The room temperature (RT) CRSS for compression twining is estimated to be between 100–150 MPa.25 In light of these data, basal slip and tensile twinning seem to be the active deformation modes in RT compression of Mg.
| Sample | Yield strength (MPa) | Tensile strength (MPa) | Ductility (%) |
|---|---|---|---|
| Transverse | 46 | 275 | 18 |
| Longitudinal | 37 | 211 | 16 |
| 45° | 43 | 213 | 19 |
The transverse sample was unfavorably oriented for basal slip, but suitably oriented for {10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 2} tensile twinning. However, as the CRSS for {10
2} tensile twinning. However, as the CRSS for {10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 2} tensile twinning is higher than basal slip, the transverse sample displays the highest yield strength. The longitudinal samples had an orientation slightly deviated from ideal basal. This orientation can lead to activation of basal slip. Since basal slip has the lowest CRSS among all the deformation modes, longitudinal samples have the lowest yield strength. The 45° sample is also favorably oriented for basal slip and consequently has lower yield strength. The ductilities of the transverse and 45° samples are higher than that of the longitudinal sample. Another important aspect is the nature of the stress–strain curve, which indicates the deformation mechanism operating during the compression test. Transverse samples display low strain hardening rate initially due to twinning. Twinning can provide a maximum compressive strain of 0.065, above which it saturates.25 The results of the present investigation are corroborated by reported literature. A low strain hardening rate can be seen from Fig. 4 at a strain around 0.065, above which it increases. Once the twining saturates, it renders the grain toward hard orientation for basal slip, leading to rapid strain hardening. At higher strains (>0.065) the strain hardening rate increases. The transverse sample initially has a lower strain hardening rate than the longitudinal sample, but increases at higher strains. As a result, the transverse curve superseded the longitudinal curve at a strain above 0.125. A higher strain hardening rate could likely be the reason for the high ductility of the transverse sample. In contrast, a lower strain hardening rate might lead to reduced ductility in the longitudinal sample. The nature of the flow curve of the 45° sample is similar to that of the transverse sample in the initial stages of plastic deformation. Both the curves indicate an initial low work hardening rate, which is likely due to the occurrence of twinning. As the strain increases, the twinning strain saturates and basal slip becomes the dominant deformation mechanism. The strain hardening, therefore, increases and the curve is similar to that of the longitudinal sample at higher strain levels. However, the effect of twinning on strain hardening is less pronounced in the 45° sample than in the transverse sample. The strain hardening rate is higher for the 45° sample than for the transverse sample. Kleiner et al. theoretically calculated the variation of twinning strain as a function of deviation from extrusion direction.19 The twinning strain in compression decreases with deviation from the initial extrusion direction. The lesser propensity of twinning in the 45° sample accounts for the difference in strain hardening rates between the 45° sample and the transverse sample. The strain hardening rate in the 45° sample at higher strain is lower than the transverse sample and similar to the longitudinal sample. Although the total strain is higher in the 45° sample than the longitudinal, the net plastic strain is same as in the longitudinal (∼0.15) sample. Similar values of ultimate tensile strength (UTS) and plastic strain can be attributed to the similar work hardening behaviors of the 45° and longitudinal samples.
2} tensile twinning is higher than basal slip, the transverse sample displays the highest yield strength. The longitudinal samples had an orientation slightly deviated from ideal basal. This orientation can lead to activation of basal slip. Since basal slip has the lowest CRSS among all the deformation modes, longitudinal samples have the lowest yield strength. The 45° sample is also favorably oriented for basal slip and consequently has lower yield strength. The ductilities of the transverse and 45° samples are higher than that of the longitudinal sample. Another important aspect is the nature of the stress–strain curve, which indicates the deformation mechanism operating during the compression test. Transverse samples display low strain hardening rate initially due to twinning. Twinning can provide a maximum compressive strain of 0.065, above which it saturates.25 The results of the present investigation are corroborated by reported literature. A low strain hardening rate can be seen from Fig. 4 at a strain around 0.065, above which it increases. Once the twining saturates, it renders the grain toward hard orientation for basal slip, leading to rapid strain hardening. At higher strains (>0.065) the strain hardening rate increases. The transverse sample initially has a lower strain hardening rate than the longitudinal sample, but increases at higher strains. As a result, the transverse curve superseded the longitudinal curve at a strain above 0.125. A higher strain hardening rate could likely be the reason for the high ductility of the transverse sample. In contrast, a lower strain hardening rate might lead to reduced ductility in the longitudinal sample. The nature of the flow curve of the 45° sample is similar to that of the transverse sample in the initial stages of plastic deformation. Both the curves indicate an initial low work hardening rate, which is likely due to the occurrence of twinning. As the strain increases, the twinning strain saturates and basal slip becomes the dominant deformation mechanism. The strain hardening, therefore, increases and the curve is similar to that of the longitudinal sample at higher strain levels. However, the effect of twinning on strain hardening is less pronounced in the 45° sample than in the transverse sample. The strain hardening rate is higher for the 45° sample than for the transverse sample. Kleiner et al. theoretically calculated the variation of twinning strain as a function of deviation from extrusion direction.19 The twinning strain in compression decreases with deviation from the initial extrusion direction. The lesser propensity of twinning in the 45° sample accounts for the difference in strain hardening rates between the 45° sample and the transverse sample. The strain hardening rate in the 45° sample at higher strain is lower than the transverse sample and similar to the longitudinal sample. Although the total strain is higher in the 45° sample than the longitudinal, the net plastic strain is same as in the longitudinal (∼0.15) sample. Similar values of ultimate tensile strength (UTS) and plastic strain can be attributed to the similar work hardening behaviors of the 45° and longitudinal samples.
3.3 Corrosion behavior
Fig. 5 shows potentio-dynamic polarization plots in Hank's solution. The Tafel extrapolation method was used to calculate corrosion potential Ecorr and corrosion current density Icorr. The values are listed in Table 3. The resorption rate of biomedical implants in vivo is a complex phenomenon influenced by a multitude of factors, including the local physiological pH, the composition of the biological milieu including salt and proteins, and transport and flow conditions. Thus, although it is difficult to mimic this environment ex vivo, the corrosion rates determined by the Tafel extrapolation method are widely accepted as a reliable measure of the expected resorption rates in vivo. The corrosion rates measured here varied with deviation of the sample surface normal from the extrusion axis. As the deviation increased, the corrosion rate was found to decrease. The corrosion rate decreased in the following order: transverse > 45° > longitudinal. It is generally believed that presence of close packed basal planes improves corrosion resistance because of their low surface energy.16,17,27 Fu et al. determined the surface energies of the (0001), (10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) and (11
0) and (11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0) planes of magnesium. The surface energy was lowest for the basal plane (1.54 × 104 J mol−1) and was higher for (10
0) planes of magnesium. The surface energy was lowest for the basal plane (1.54 × 104 J mol−1) and was higher for (10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) and (11
0) and (11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0) at 3.04 × 104 J mol−1 and 2.99 × 104 J mol−1, respectively.17,28 Table 2 also lists the volume fraction of basal fibers. The volume fraction is low for both the transverse and 45° samples and, thus, these show high corrosion rates. Interestingly, despite having a lower basal fiber volume fraction, 45° showed a lower corrosion rate than transverse. This is likely due to the fact that planes parallel to the 45° surface are closer to basal orientation than those of the transverse sample (see IPF in Fig. 3). The results are in agreement with previous reports on the effect of texture on the corrosion behavior of magnesium alloys. Xin et al.16 studied the effect of texture on the corrosion behavior of extruded AZ31 alloy in 3.5 wt% NaCl.16 They observed a continuous decrease in corrosion rate with an increase in volume of grain with basal orientation. Song et al.17 predicted from theoretical studies that that the corrosion rate of the (0001) planes is approximately twenty times lower than those of the (10
0) at 3.04 × 104 J mol−1 and 2.99 × 104 J mol−1, respectively.17,28 Table 2 also lists the volume fraction of basal fibers. The volume fraction is low for both the transverse and 45° samples and, thus, these show high corrosion rates. Interestingly, despite having a lower basal fiber volume fraction, 45° showed a lower corrosion rate than transverse. This is likely due to the fact that planes parallel to the 45° surface are closer to basal orientation than those of the transverse sample (see IPF in Fig. 3). The results are in agreement with previous reports on the effect of texture on the corrosion behavior of magnesium alloys. Xin et al.16 studied the effect of texture on the corrosion behavior of extruded AZ31 alloy in 3.5 wt% NaCl.16 They observed a continuous decrease in corrosion rate with an increase in volume of grain with basal orientation. Song et al.17 predicted from theoretical studies that that the corrosion rate of the (0001) planes is approximately twenty times lower than those of the (10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) or (11
0) or (11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0) prismatic planes.
0) prismatic planes.
| Sample | Corrosion potential Ecorr (mV vs. SCE) | Corrosion current density Icorr (mA cm−2) | Basal fiber volume fraction (%) |
|---|---|---|---|
| Transverse | −1667 | 0.16 | 0.1 |
| Longitudinal | −1645 | 0.05 | 8.2 |
| 45° | −1631 | 0.10 | 0.1 |
3.4 Biocompatibility test
The effect of texture on the viability of osteoblasts was evaluated through an indirect method wherein cells were cultured in conditioned medium after exposure to magnesium samples with different textures. Fig. 6 shows the result of the MTT assay at 1 day and 3 days after adding conditioned medium. After 1 day, there was no statistically significant difference between the three samples and fresh medium, which served as the control. By day 3, the cells had proliferated in all four conditions. Cell viability was significantly higher in the control compared to the three media exposed to Mg samples. However, no statistical difference was observed among the three conditioned media. Thus, it appears that cytocompatibility was largely unaffected by the texture of magnesium. | ||
| Fig. 6 Absorbance values of MTT assay after 1 day and 3 days. * represents a statistically significant difference (p < 0.05) after 3 days. | ||
The anodic and catholic reactions that occur during corrosion of magnesium are as follows:
| Anodic: Mg → Mg2+ + 2e− |
| Cathodic: 2H2O + 2e− → H2 + 2OH− |
Corrosion leads to simultaneous increase in Mg2+ concentration and the pH of the medium due to the formation of OH− ions. The pH values of all the three conditioned media were similar at 8.0 (Table 4). The values of magnesium concentration present in the media conditioned for 24 h measured by AAS are also listed in Table 4. There was no statistically significant difference in concentration among the three samples. Theoretically, the transverse and longitudinal samples should have much higher corrosion rates than the 45°. However, there was no measurable difference in the Mg2+ concentration when the samples were conditioned in growth medium. Mg(OH)2 along with other salts will precipitate following Mg corrosion in the culture medium. All such precipitates were removed by centrifugation and only dissolved Mg2+ ions remained in the conditioned medium for the cell studies. Moreover, an initial high corrosion rate of the transverse sample might have led to the formation of a protective Mg(OH)2 layer, retarding its further corrosion. This does not however mean that Mg2+ concentration is stabilized after 24 h. Mg2+ concentration will increase with time but the rate will be slower due to the presence of the partially protective Mg(OH)2 layer. Conditioned medium was extracted after 24 h to compare the effect of texture on biocompatibility. The conditions are likely to be different in vivo due to the presence of dynamic conditions where the corrosion products will be transported away by biological fluids. Therefore, it is important to note here that Mg2+ concentration in the conditioned medium should not be considered as a true measure of resorption rate. Corrosion rate determined by the Tafel extrapolation method is a more reliable indicator of the resorption rate.
| Sample | pH of the conditioned media | Mg2+ concentration in conditioned media (μg ml−1) |
|---|---|---|
| Transverse | 8.1 ± 0.1 | 766.0 ± 90.0 |
| Longitudinal | 8.2 ± 0.1 | 850.0 ± 53.5 |
| 45° | 8.1 ± 0.1 | 837.5 ± 76.7 |
The results observed for the biocompatibility tests were investigated further. Immersion of magnesium in growth medium leads to aqueous corrosion. It is possible that either increased Mg2+ or pH in conditioned medium affected cell viability. The individual effects of Mg2+ concentration and pH on osteoblast viability were studied. Growth medium was replaced with medium having known Mg2+ concentration and pH. The effect on osteoblast viability was measured after 1 day and 3 days to compare with the effect of conditioned medium on viability after 1 day and 3 days respectively. Fig. 7(a) shows the effect of Mg2+ on cell viability at 1 day and 3 days after replacing growth medium with medium having a known concentration of Mg2+. The concentration was varied from 20 μg ml−1 (growth medium) to 104 μg ml−1. It was observed that after 1 day the cell viability was not affected for Mg2+ concentrations up to 4 × 103 μg ml−1. On the other hand, a concentration of 104 μg ml−1 was found to be toxic as all the cells died within 1 day. Cells were able to proliferate at all concentrations lower than 104 μg ml−1. However, the proliferation rate decreased with an increase in concentration. The cell viability was statistically similar to the control at concentrations up to 750 μg ml−1 but lower at higher concentrations. Thus, concentrations of Mg2+ up to 4 × 103 μg ml−1 are mildly toxic to osteoblasts although the effect is inhibited cell growth. Extremely high concentration (>104 μg ml−1) is toxic to osteoblasts. Hallab et al. found Mg mildly toxic to MG-63 cells at 50 mM (1250 μg ml−1) Mg2+ concentration.29
The effect of pH on cell viability and proliferation is shown in Fig. 7(b). It was observed that after 1 day the cell viability was statistically similar to that of the control (pH = 7.4) up to pH 9.0. The cell viability was significantly lower than the control for pH 10.0, whereas all the cells died at pH levels greater than 10.0. After 3 days the cells proliferated at all pH levels except pH 11.0 and 12.0, where all the cells had died within 1 day. However, the proliferation rate slowed with an increase in pH. The cell viability at 3 days was statistically similar for the control and pH 8.0, but was statistically lower than the control for higher pH values. Thus, these data indicate that although higher pH (<10) inhibits cell growth, it is not cytotoxic. However, extremely high pH (>10) leads to complete cell death. The results are in agreement with the work of Gu et al., where extracts of binary Mg alloys with pH levels around 8.8 did not significantly reduce the viability of MC3T3-E1 osteoblasts.9 From the results of the pH experiment (Fig. 7(b)), it can be said that pH did not play a role in slowing the cell growth in 3 days for all the three samples. The Mg2+ concentration in all three conditioned media extracted after 24 h of sample immersion was approximately 800 μg ml−1. This value is below the limit which can retard osteoblast proliferation (Fig. 7(a)). However, the observed slower proliferation rates in the samples compared to the control could likely be the combined effect of relatively higher pH and Mg2+ concentration. It must be noted that the biocompatibility tests in this study were based on an indirect assay in static culture. The implants in the human body experience a more complex and dynamic environment. Body fluids will transport both precipitated Mg salts as well as dissolved Mg2+ ions away from the implant site. The cytocompatibility will be influenced by the transport of corrosion products with the fluids. Therefore, further studies with animal models are warranted to fully evaluate the role of texture on the biocompatibility of magnesium implants.
Few studies have reported the effect of crystallographic texture on the performance of biomaterials. Faghihi et al.22 showed that texture of Ti-6A-4V alloy affects osteoblast attachment and proliferation through changes in surface energies. In another study, Hoseini et al.21 found that a higher number of Ti–OH bonds are facilitated by presence of close packed planes which indeed improved cell–material interaction. In a recent study, we reported a systematic study on the role of texture in controlling the mechanical properties, corrosion behavior and osteoblast response to titanium.20 Even as magnesium has emerged as a promising candidate for use as a resorbable biomaterial, there is little understanding of the role, if any, of texture on its performance. From the findings of this study, it can be seen that texture influenced mechanical properties and corrosion resistance without a significant change in cytocompatibility. Thus, texture can be utilized to tune the mechanical properties and resorption rates without the loss of biocompatibility while designing magnesium-based resorbable implants, in addition to other strategies such as alloying, processing and surface modification.
4. Conclusion
The effect of texture on the mechanical properties, corrosion behavior and biocompatibility of pure magnesium in extruded conditions was systematically evaluated. Texture significantly affected the mechanical properties. The samples taken perpendicular to the extrusion direction had higher strength than those extracted along 45° or parallel to the extrusion direction. Texture also affected ductility, which was also the highest along the sample taken perpendicular to the extrusion direction. The corrosion rate in Hank's solution was found to vary in the following decreasing order: transverse, at 45°, and along the extrusion direction. These characteristics have been attributed to the presence of basal planes at the surface, which varies with texture and in turn controls the corrosion rates. Resistance to corrosion could be scaled with increasing fraction of grains with basal planes parallel to the surface. On the other hand, for pure Mg, biocompatibility was found to be less sensitive to texture. Taken together, this study suggests that the mechanical properties and resorption rates of magnesium implants may be tuned through control of texture without loss of biocompatibility. Although texture has been utilized to improve the properties of Mg as structural material, this is the first comprehensive study demonstrating its application in modulating properties of Mg for use as a resorbable biomaterial.Acknowledgements
This work was funded by the Department of Atomic Energy-Board of Research in Nuclear Sciences (DAE-BRNS). K.C. was financially supported by the Ramanujan fellowship from Department of Science and Technology (DST), India. Authors gratefully acknowledge Prof. Subodh Kumar and Mr George Raphael for assistance with the corrosion studies and Mr R. Deshpande for help with AAS.References
- M. P. Staiger, A. M. Pietak, J. Huadmai and G. Dias, Biomaterials, 2006, 27, 1728–1734 CrossRef CAS PubMed.
- O. Bostman and H. Pihlajamaki, J. Trauma, 1996, 41, 846–849 CrossRef CAS PubMed.
- B. O’Brien and W. Carroll, Acta Biomater., 2009, 5, 945–958 CrossRef PubMed.
- B. Heublein, R. Rohde, V. Kaese, M. Niemeyer, W. Hartung and A. Haverich, Heart, 2003, 89, 651–656 CrossRef CAS.
- F. Witte, Acta Biomater., 2010, 6, 1680–1692 CrossRef CAS PubMed.
- G. Manivasagam and S. Suwas, Mater. Sci. Technol., 2014, 30, 515–520 CrossRef CAS PubMed.
- R. W. Li, N. T. Kirkland, J. Truong, J. Wang, P. N. Smith, N. Birbilis and D. R. Nisbet, J. Biomed. Mater. Res., Part A, 2014, 102, 4346–4357 CAS.
- M. B. Kannan and R. Raman, Biomaterials, 2008, 29, 2306–2314 CrossRef CAS PubMed.
- X. Gu, Y. Zheng, Y. Cheng, S. Zhong and T. Xi, Biomaterials, 2009, 30, 484–498 CrossRef CAS PubMed.
- Z. Li, X. Gu, S. Lou and Y. Zheng, Biomaterials, 2008, 29, 1329–1344 CrossRef CAS PubMed.
- E. Zhang and L. Yang, Mater. Sci. Eng., A, 2008, 497, 111–118 CrossRef PubMed.
- Y. Xin, T. Hu and P. Chu, Acta Biomater., 2011, 7, 1452–1459 CrossRef CAS PubMed.
- G. Argade, S. Panigrahi and R. Mishra, Corros. Sci., 2012, 58, 145–151 CrossRef CAS PubMed.
- L. Zhao, C. Cui, Q. Wang and S. Bu, Corros. Sci., 2010, 52, 2228–2234 CrossRef CAS PubMed.
- L. Li, J. Gao and Y. Wang, Surf. Coat. Technol., 2004, 185, 92–98 CrossRef CAS PubMed.
- R. Xin, B. Li, L. Li and Q. Liu, Mater. Des., 2011, 32, 4548–4552 CrossRef CAS PubMed.
- G.-L. Song, R. Mishra and Z. Xu, Electrochem. Commun., 2010, 12, 1009–1012 CrossRef CAS PubMed.
- J. Del Valle, F. Carreno and O. Ruano, Acta Mater., 2006, 54, 4247–4259 CrossRef CAS PubMed.
- S. Kleiner and P. Uggowitzer, Mater. Sci. Eng., A, 2004, 379, 258–263 CrossRef PubMed.
- S. Bahl, S. Suwas and K. Chatterjee, RSC Adv., 2014, 4, 38078–38087 RSC.
- M. Hoseini, P. Bocher, A. Shahryari, F. Azari, J. A. Szpunar and H. Vali, J. Biomed. Mater. Res., Part A, 2014, 102, 3631–3638 CrossRef PubMed.
- S. Faghihi, F. Azari, H. Li, M. R. Bateni, J. A. Szpunar, H. Vali and M. Tabrizian, Biomaterials, 2006, 27, 3532–3539 CAS.
- H. Sudo, H.-A. Kodama, Y. Amagai, S. Yamamoto and S. Kasai, J. Cell Biol., 1983, 96, 191–198 CrossRef CAS.
- W. Hutchinson and M. Barnett, Scr. Mater., 2010, 63, 737–740 CrossRef CAS PubMed.
- A. Chapuis and J. H. Driver, Acta Mater., 2011, 59, 1986–1994 CrossRef CAS PubMed.
- C. M. Byer, B. Li, B. Cao and K. Ramesh, Scr. Mater., 2010, 62, 536–539 CrossRef CAS PubMed.
- M. Hoseini, A. Shahryari, S. Omanovic and J. A. Szpunar, Corros. Sci., 2009, 51, 3064–3067 CrossRef CAS PubMed.
- B.-Q. Fu, W. Liu and Z.-L. Li, Appl. Surf. Sci., 2009, 255, 9348–9357 CrossRef CAS PubMed.
- N. J. Hallab, C. Vermes, C. Messina, K. A. Roebuck, T. T. Glant and J. J. Jacobs, J. Biomed. Mater. Res., 2002, 60, 420–433 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |