A disposable biofilm-modified amperometric biosensor for the sensitive determination of pesticide biotoxicity in water†
Jun Qianab,
Jiuming Lia,
Deyu Fanga,
Yuan Yu*a and
Jinfang Zhi*a
aKey Laboratory of Photochemical Conversion and Optoelectronic Materials, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, Beijing 100190, China. E-mail: yyu@mail.ipc.ac.cn; zhi-mail@mail.ipc.ac.cn
bCAS Key Laboratory of Soft Matter Chemistry, Department of Chemistry, University of Science and Technology of China, Hefei, Anhui 230026, China
First published on 7th October 2014
Abstract
In the present study, a disposable biofilm-modified amperometric microbial sensor was developed and employed as an analytical tool for evaluating the biotoxicity of pesticides and real wastewater. The proposed biotoxicity biosensor was fabricated by using a polymeric disposable biofilm, which was prepared by immobilizing the pretreated S. cerevisiae cells (as a highly active biocatalyst) on a combination of polyvinyl alcohol (PVA) hydrogel and sodium alginate crosslinked by CaCl2 as a matrix at the electrode surface. It was found that the S. cerevisiae pretreated with alcohols is needed for increasing the response sensitivity of the microbial sensor; therefore, the effects of the treated reagents, concentration and treating time, etc., on the performance of the biosensor were optimized. Atomic force microscopy (AFM), flow cytometry (FCM), as well as the electrochemical response, were employed to investigate the morphology characteristics, cells viability and electrochemical characteristics, respectively. 3,5-Dichlorophenol (DCP) was taken as the reference toxicant. Pesticide solutions, including Acephate, Ametryn and Thiram were selected as model toxicants. The biotoxicity of four kinds of real wastewater was also determined by this amperometric microbial sensor. The traditional parameter of 50% inhibitory concentration (IC50) was measured within 30 min and the results obtained were better than those of other toxicity bioassays reported. The microbial biosensor prepared is a sensitive, rapid, convenient and cheap alternative to toxicity screening of chemicals and real wastewater.
1. Introduction
Pesticides are widely used for the control of pests in the environment, and may exert a significant effect, especially on aquatic ecosystems.1 Conventional physical/chemical analysis methods can only quantify the concentrations of individual pollutants. In recent years, a large number of laboratory models of biotoxicity analyzers have been described, using plants, invertebrates, daphnids and luminescence bacteria as test organisms,2–5 but most of these bio-based assays are time-consuming, complex and must be employed off-line or using expensive instruments. Electrochemical biosensors detect the analytes more rapidly and sensitively in comparison to conventional techniques, but the more typical problems in the development of electrochemical biotoxicity bioassays are how to increase the sensitivity and prolong the lifetime of the biomaterials, and also to develop broad spectrum assays for toxic substances. Efforts have been focused on the development of novel biosensors that would provide a stable, simple, rapid and on-line response for monitoring and detecting water pollution or potential risk to human health.For the electrochemical bioassay, whole cells are excellent indicators for a variety of parameters, in particular for toxic compounds. They can respond unspecifically and thus represent an excellent means for determining the overall toxicity of pollutants. In recent studies, most whole cell biosensors that have been reported are based on bacterial cells such as E. coli, Bacillus subtilis and Pseudomonas putida.6–8 However, there are also some disadvantages in their use. For example, prokaryotic bacterial cells may be relatively weak in sensor environments, leading to short lifetimes and limited pH, osmotic and temperature tolerances of individual species, which means that the operating parameters of the sensor could also be limited.
As eukaryotes occupy a wide range of environmental niches, their determination results are more reliable and they are good models for assessing toxicity compared to prokaryotic bacterial cells. Two reviews have outlined the advantages of using yeasts in sensing applications.9,10 Parry noted the physical robustness of these yeasts in comparison to bacteria, and superior pH, temperature and osmolarity/ionic strength tolerances.9 Walmsley et al. in their review gave two further reasons to use yeast in whole cell sensors.10 The first is an advantage shared with bacteria: rapid growth, ease of manipulation and growth on a broad range of substrates; the second advantage, also noted by Parry,9 is that these organisms are eukaryotes and can sometimes provide information of direct relevance to other eukaryotes that prokaryotic cells cannot.
Despite these advantages, the number of yeast species used in toxicity applications to date is small compared to the number of bacterial cells that have been exploited.11,12 This may be due to the permeability barrier of the cell envelope for substrates and products, which often causes very low reaction rates for whole cells.13 Therefore, it is important to develop an effective method to reduce the permeability barrier and to prepare whole cell biocatalysts with high activities. Garjonyte et al. reported that the permeabilization of yeast cells could achieve significantly higher electrode responses compared to the corresponding intact yeast cells.14 Common permeabilization methods include cell treatment with solvents, detergents, salts, cell freezing and thawing or electro-permeabilization, etc.15,16 To the best of our knowledge, a mild cell-wall permeabilization technology by alcohols aimed at S. cerevisiae used for biotoxicity assays has not been reported.
Moreover, during the fabrication process of the electrochemical whole cells biosensor, coating microorganism cell suspensions on the electrode's surface is typically used,17,18 but its shortcoming is that the surfaces of electrodes could be easily contaminated during the electrochemical biotoxicity assay. While an electrode with a disposable microbial film can avoid inaccurate data caused by secondary contamination of the film in the repeat detection biotoxicity process, it is also convenient for the next biotoxicity determination through changing to a new film. In particular, such single-used probes are advantageous in polluted water and complex media; thus, the fabrication of a low-cost and disposable electrode for biosensors is of vital importance.
Therefore, we report for the first time the development of a single-use biofilm modified amperometric microbial sensor for the sensitive on-line determination of the biotoxicity of pesticides in water. Remarkably, the S. cerevisiae cells were pretreated with alcohols to increase the permeability of their cell walls, which could effectively improve the determination sensitivity for organic pesticides and pollutants. The pretreatment effects on S. cerevisiae cells were evaluated by the combination of AFM images and FCM, as well as by the electrochemical responses. Furthermore, the amperometric biosensor was fabricated from a single-use biofilm modified Pt electrode, which was prepared by immobilizing pretreated S. cerevisiae by using a complex of PVA solution and sodium alginate solution crosslinked with CaCl2, which is porous enough to allow the diffusion of substrates to the cells. The feasibility of the proposed bioassays in determination of pesticide toxicity is further successfully demonstrated using three different kinds of pesticide, Ametryn, Acephate and Thiram, and the IC50 values are determined to be 22.0 mg L−1, 29.0 mg L−1 and 47.5 mg L−1, respectively. According to the IC50 values, the toxicity, in descending order, is Ametryn > Acephate > Thiram. 3,5-Dichlorophenol (DCP) was taken as a reference toxicant and its IC50 value is 9.83 mg L−1. Finally, the biotoxicities of four kinds of real wastewaters were also determined. All the above determination results show that this prepared, disposable, S. cerevisiae biofilm-modified amperometric microbial sensor can effectively determine the biotoxicity of chemical toxicants.
2. Experimental
2.1. Reagents
A cocktail of respiratory substrates (sodium lactate, sodium succinate and glucose, each at 10 mM) in 0.85% saline was used for regenerating and monitoring the biosensors. p-Benzoquinone (BQ) was used as the mediator. All chemicals were of analytical grade (Beijing Lanyi Chemical Products Co., Ltd., China). All toxicant (3,5-DCP, Acephate, Ametryn, Thiram) solutions at the desired concentrations were prepared by diluting their stock solutions with deionized water. Stock solutions were prepared fresh or kept at 4 °C. Low solubility compounds were dissolved with the help of 0.5% (v/v) of dimethyl sulfoxide (DMSO).19 The selected toxicants possess different chemical structures, for example, Ametryn, Acephate and Thiram are a triazine weedkiller, organophosphorus insecticide and sulfur fungicide, respectively, which are widely used and thus chosen as target toxicants. The three kinds of real wastewaters were taken from a garbage-treatment plant, an electroplating factory and a laboratory, without any treatment. The used real wastewaters, containing organics, inorganics or both, are our selecting basis for wastewater, in order to reflect the broader spectrum responses to water samples by our prepared sensor.2.2. Microorganism preparation
S. cerevisiae S288C was obtained from China General Microbiological Culture Collection Center (CGMCC). Rich medium (YEPD) contained 2% glucose, and 1% yeast extract. A 100 mL solution of autoclaved YEPD medium was inoculated with a colony of S. cerevisiae and grown aerobically at 30 °C for 24 h on a rotary shaker (200 rpm). Then S. cerevisiae cells were harvested by centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min at room temperature, washed twice with PBS and resuspended in PBS. The final concentration of cells was adjusted to an absorbance value of 24.0, measured at 600 nm using a Secoman Uvikon UV-Vis Spectrophotometer. The S. cerevisiae suspension was stored at 4 °C until required.
000 rpm for 10 min at room temperature, washed twice with PBS and resuspended in PBS. The final concentration of cells was adjusted to an absorbance value of 24.0, measured at 600 nm using a Secoman Uvikon UV-Vis Spectrophotometer. The S. cerevisiae suspension was stored at 4 °C until required.
The S. cerevisiae pretreatment procedures were to firstly incubate the untreated S. cerevisiae cells in different concentrations (20, 30, 40%, v/v) of methanol or ethanol solutions in phosphate buffer. Then these pretreated cell suspensions were centrifuged and washed with sterile deionized water three times. To find the optimized pretreatment effect, electrode responses to the acute biotoxicity of 10 mg L−1 DCP were tested and repeated three times.
2.3. Morphology studies by AFM
The AFM samples were prepared by spreading 100 μL of the pretreated yeast cell suspensions onto the surfaces of glass slides, and then leaving them to dry for 30 min at room temperature. All images were obtained from a ScanAsyst-AIR mode atomic force microscope (AFM, BRUKER MultiMode® 8, GER). A rotated (symmetric) silicon tip/a triangular silicon nitride cantilever with a spring constant of 0.4 N m−1 and a resonance frequency of ∼70 kHz was used. The scan speed was set at 0.7 Hz and the final resolution was 256 by 256 pixels. Each scan resulted in a topography image and a phase image simultaneously. The height scale of the cell was depicted as shades of gray, with bright areas being nearer to the tip in topography images. All the AFM experiments were performed at room temperature.2.4. Viability of yeast cells determined by FCM
S. cerevisiae cell suspensions (pretreated or untreated) were incubated in 50 μg L−1 propridium iodide (PI) at room temperature for 5 min. Viability was analyzed on a FACScan flow cytometer (FCM, BD Biosciences, San Jose, CA) equipped with a 15 mW air-cooled 488 nm argon ion laser for excitation of PI. At least 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells were analyzed for each sample.
000 cells were analyzed for each sample.
2.5. Preparation of disposable biofilm and characterization by SEM
100 μL of 10% (w/v) PVA (degree of saponification 98%, degree of polymerization 2400) aqueous solution was thoroughly mixed with 10 μL of 1.0 M sodium sulfate aqueous solution and 40 μL of 0.8% (w/v) sodium alginate aqueous solution (ESI Fig. S1†). The treated yeast cell suspension was centrifuged at 5000 rpm for 10 min at room temperature, and then washed twice with pH 7.0 PBS before use. Finally, the 50 μL washed cell suspension was added to the above mixture solution. The resulting mixture was spread over the surface of a polyethylene terephthalate (PET) plastic sheet by spin coating so as to distribute it well. The PET sheet coated with yeast cells was further dripped in 2% (w/v) calcium chloride solution and crosslinked for 15–20 min, forming the PVA–alginate microbial biofilm of 0.05–0.10 mm thickness. The prepared biofilms were peeled off from the PET sheet and washed thoroughly with PBS and stored at 4 °C until use. The surface morphology of the prepared microbial films was investigated by SEM (Hitachi Ultra-High-Resolution S-4300).2.6. Electrochemical measurements and toxicity assay
The electrochemical experiments were performed with a potentiostat/galvanostat (Model 263 A, Princeton, USA). Electrochemical impedance spectroscopy (EIS) measurements were carried out with a 263A potentiostat/galvanostat and a FRD 100 frequency response detector (Princeton, USA). The electrochemical measurements were carried out in a biofilm reactor (BFR), including with the microbial working electrode, a platinum wire auxiliary electrode, and a Ag/AgCl (saturated KCl) reference electrode, respectively. The working electrode was obtained by attaching and fixing an appropriate size of the biofilm described above on the surface of a platinum disk through a rubber O-ring (Scheme 1a).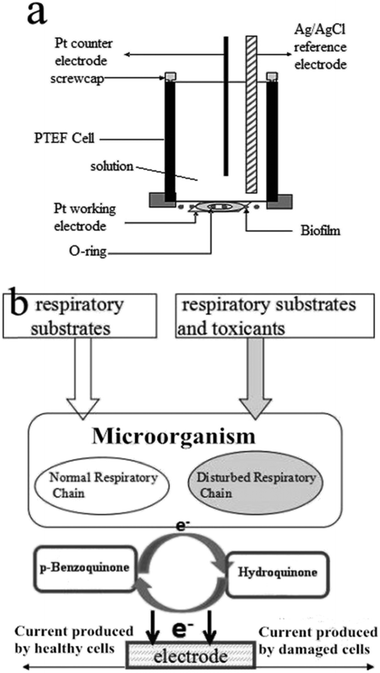 | ||
| Scheme 1 (a) Schematic diagram of the disposable microbial film sensor. (b) Principle of the BQ-mediated biotoxicity assessment. | ||
The biosensors were monitored in a stirred vial (700 rpm) containing 10 mL pH 7.0 respiratory substrates solution by applying a potential of 0.3 V (vs. Ag/AgCl) at room temperature, and the current was monitored and data were displayed in real-time as current against time plots. After a stabilization period of about 5 min, 100 μL of BQ solution as a redox mediator was added to the respiratory substrates to produce a final concentration of 0.4 mM. Target toxicants were added in the same way after the second period of stabilization. After adding a toxicant sample, the current was decreased because of their detrimental effect to the metabolic activity of microorganisms. The toxicity can be determined by measuring changes of the BQ-mediated respiration chain activity. As shown in Scheme 1b, BQ can be reduced to hydroquinone (HQ) during cell respiration, and the resultant HQ can be reoxidized to BQ at the electrode surface. For each toxicant concentration, the anodic currents were converted to equivalent inhibitory percentage values according to eqn (1):
| Inhibition% = (1 − I2/I1) × 100% | (1) |
2.7. EIS characterization of the disposable biofilm on the Pt electrode
We are aiming to develop a single-use, disposable biofilm to modify the Pt electrode, so the electron transfer properties of the interface between the Pt electrode and the biofilm become important. EIS characteristics were performed when the biofilm was attached and fixed on the Pt surface because it is capable of monitoring the adhesion of biofilms with the Pt electrode and measuring the resulting change in the electron-transfer resistance. As controls, a duplicate electrochemical sensor constructed using a bare Pt electrode with no biofilm, as well as a conventional non-disposable biofilm modified Pt electrode which was obtained by directly coating the PVA/pretreated S. cerevisiae gel onto the surface of the Pt electrode, were used to compare the differences in electron transfer processes.3. Results and discussion
3.1. Optimization of the pretreatment conditions of S. cerevisiae cells
S. cerevisiae cells, as a micro-organism used in the present biosensor, have thick and strong cell walls which cause very low biocatalytic activity of whole cell biosensors. It is important to develop an effective method to reduce the permeability barrier of the cell wall. During the pretreatment, particular care has to be taken in order to prevent loss of cell viability; considering efficiency and cost factors, alcohol solvents are considered the most adequate treatment agents for S. cerevisiae cell permeabilization.14 In the present study, methanol and ethanol were selected as permeabilizing agents for S. cerevisiae cells. To optimize the pretreatment conditions, the alcohol species, concentration and treating time were investigated by AFM and FCM.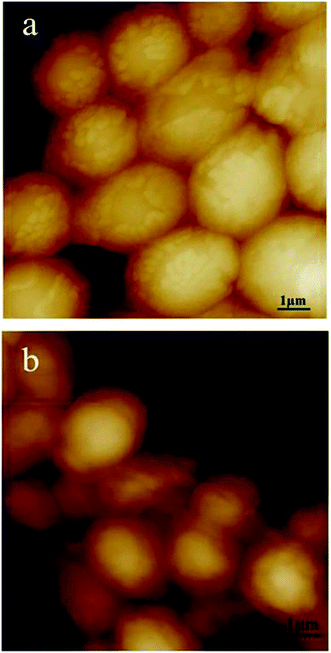 | ||
| Fig. 1 AFM images of S. cerevisiae cells pretreated by ethanol at different concentrations. (a) 30% (v/v) ethanol, 16 h; (b) 40% (v/v) ethanol, 16 h. | ||
S. cerevisiae cells grown in different methanol concentrations were also examined. The effect of the permeabilization at lower concentrations (20%) for 16 h was similar to those at 20% ethanol, i.e., no obvious changes were observed (ESI Fig. S2d†); when cells were exposed to 30% and 40% (v/v) methanol for up to 16 h it could be observed that most of the cell walls peeled off and the cells almost became protoplasts (ESI Fig. S2e and f†). Moreover, some cell shapes became irregular, compared to those of the control cells.
 | ||
| Fig. 2 Viability of S. cerevisiae cells in methanol and ethanol (20%, 30%, 40% (V/V)) after 6 h (black) and 16 h (gray) treatment. Bar: mean ± SD (n = 3). | ||
So combining the results of the AFM imaging (Fig. 1) and the viability experiments of the cells, 30% (v/v) ethanol and a treatment time of 16 h were set as the optimal conditions for further studies. This was in accordance with the findings of Garjonyte.14 They considered that the high activity of S. cerevisiae treated by ethanol was probably largely attributable to increased water permeability of S. cerevisiae membrane.
3.2. Characterization of PVA–Ca alginate/yeast cells disposable biofilm
 | ||
| Fig. 3 (a) The low-magnification SEM image of the PVA–Ca alginate biofilm enclosed with the pretreated S. cerevisiae. (b) The magnified image of (a). | ||
Additionally, it is important to mention that sodium sulfate induced crystallite formation in PVA as sulfate ions possess the ability to form linkages among PVA.24 In the PVA–sodium sulfate hydrogel preparation, the sulfate ions quickly penetrated into the PVA hydrogel solution and destabilized hydrogen bonding between hydroxyl groups of PVA and H2O by polarizing the water molecules.25 As a result, the formation of hydrogen bonds between the hydroxyl groups of PVA was facilitated, resulting in PVA crystallite formation.22 XRD patterns of PVA–sodium sulfate hydrogel, dried PVA powder, dried sulfate hydrogel powder and PVA hydrogel are shown in ESI Fig. S3.† Finally, we found that this crystallite formation can effectively overcome the swelling of the hydrogel matrix.
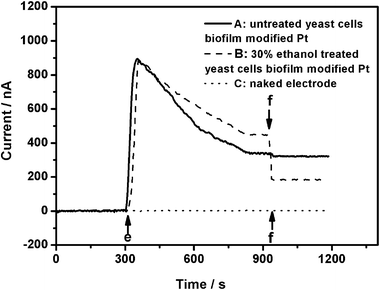
As shown in Fig. 4, the analysis process was as follows: (1) the biosensor operation was carried out in a stirred vial containing 10 mL pH 7.0 respiratory substrates solution by applying a potential of 0.3 V (vs. Ag/AgCl) at room temperature; (2) following a biosensor stabilization period I of about 5 min, 100 μL of p-benzoquinone solution as a redox mediator (as shown in Fig. 4 position e) was injected to a final concentration of 0.4 mM. Then the current first began to increase significantly, followed by a decrease and finally reached a second period of stabilization II of about 5–10 min and noted as I1 (baseline current); (3) when the toxicant samples were treated in the same way in the second stabilization period II (Fig. 4, position f), the current was monitored and noted as I2; (4) the current data were displayed in real-time as current against time plots; (5) then, the anodic currents were converted to equivalent inhibitory percentage values according to eqn (1): inhibition% = (1 − I2/I1) × 100%.
It was found that there were no obvious changes of the current when injecting 100 μL if p-benzoquinone (position e, dotted line, Fig. 4) followed by 100 μL of 10 mg L−1 DCP (position f, dotted line, Fig. 4) on the naked Pt electrode. It is well known that mediators fundamentally work by interacting with the metabolic pathways of the biocatalyst. The reduction activity of a mediator is a sensitive indicator of xenobiotic toxicity to microorganisms because it is directly coupled to respiration via the electron transport chain, and decreases when noxious substances are present.27 BQ can accept electrons from cells easily, which was proved clearly by the immediate increase of anodic current when injecting BQ (position e, Fig. 4) on the electrode modified by the 30% ethanol-pretreated S. cerevisiae cells 16 h disposable biofilm. Compared to the untreated S. cerevisiae cells disposable biofilm-modified Pt electrode (solid line), when injecting 10 mg L−1 DCP, the pretreated cells biofilm-modified Pt electrode response curves, as expected, showed a large current decline (position f, dashed line, Fig. 4). For biosensor A and biosensor B, the inhibition values to S. cerevisiae of DCP are 4.82% and 57.78%, respectively, according to eqn (1). These results showed that the respiratory activity of S. cerevisiae can be inhibited effectively by DCP, and the permeabilized S. cerevisiae cells are more sensitive to DCP than the untreated S. cerevisiae cells. It further suggests that the permeabilized S. cerevisiae cells should be useful as a sensitive bio-component for electrochemical biotoxicity assays.
Fig. 5 shows the inhibition curves of the pretreated yeast cells with different concentrations of methanol or ethanol for different times with 10 mg L−1 DCP. Whether treated with methanol or ethanol, the sensitivities of the electrodes increased when their concentrations of alcohol were lower than 30%. However, the maximum response values of the electrodes that contained cells treated with 30% ethanol were much higher than those treated with 30% methanol. When the concentration reached 40% and the treatment time was more than 16 h, the responses sensitivities decreased. Therefore, when yeast was pretreated with 30% ethanol for 16 h, the most sensitive and stable responses were obtained. This was consistent with the AFM results. We speculated that the reason for the high response sensitivity is that the mediator could reach the enzyme in the cells and membrane permeabilization was also favorable for DCP penetration into the yeast cells.
3.3. EIS characterization of the disposable biofilm on the Pt electrode
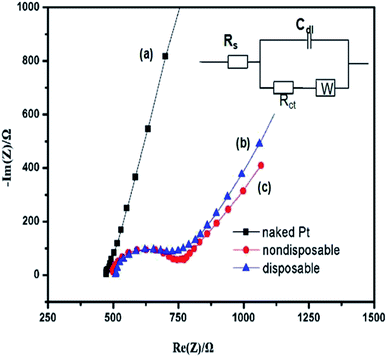
A Nyquist plot commonly includes a semicircle region lying on the axis followed by a straight line. The semicircular part seen at higher frequencies corresponds to the electron transfer-limited process and its diameter is equal to the charge transfer resistance (Rct), which controls the electron transfer kinetics of the redox probe at the electrode interface. Fig. 6 displays EIS observed for bare Pt, nondisposable biofilm-modified Pt, and a disposable biofilm/Pt in 10 mM [Fe(CN)6]3−/4−, in 0.1 M KCl from 100 KHz to 10 Hz. The inset in Fig. 6 shows the most frequently used equivalent circuit for modeling the EIS experiments, Randles equivalence circuit model, which contains the electrolyte resistance (RS) of the bulk solution in series with double-layer capacitance (Cdl), charge transfer resistance (Rct), and Warburg impedance (ZW). Compared to the bare Pt electrode (curve a), the Nyquist plots of both electrodes modified by biofilms all exhibited the characteristic semicircles at high frequencies and a straight line at low frequencies, corresponding to kinetic and diffusion processes, respectively. The Rct of the nondisposable biofilm/Pt electrode (curve c) was estimated to be 206 Ω; however, the Rct of disposable biofilm/Pt electrode (curve b) displayed a lower value of 185 Ω. These results imply that, firstly, as expected, the attachment of the biofilms on the metal surface retards the interfacial electron-transfer kinetics and increases the electron-transfer barrier between the redox analytes (p-benzoquione in the present study) and the electrode surface; and moreover, the similar EIS behavior of the two biofilm-modified Pt electrodes suggests that attaching the disposable biofilm onto the Pt surface shows no difference to the conventional biofilm modified Pt in electrochemical characteristics, despite a slight increase in Rct. Therefore, the present result is encouraging, as the disposable biofilm was shown to compare well with the conventional nondisposable biofilm electrode and, over the range of data presented, demonstrated a useable response.3.4. Toxicity assessment
Mediated, amperometric, microbial biosensors have been developed for toxicity assessment.5 In the present study, AFM observations, electrochemical responses and FCM results showed that the optimized pretreatment conditions were 30% (v/v) ethanol for 16 h. Therefore, we selected 30% (v/v) ethanol for yeast pretreatment for 16 h in the following biotoxicity tests. BQ was used as an electron transfer mediator and the pretreated S. cerevisiae cells were loaded into the PVA gel as the biological component. After being activated, the biofilm fabricated by the PVA gel/pre-treated S. cerevisiae was attached and fixed onto the Pt electrode to construct a disposable amperometric biosensor for the biotoxicity assay. This biosensor with a disposable microbial film can avoid inaccurate data caused by film pollution in repeat detection of biotoxicity processes.The responses of the biosensor operated under optimal conditions (i.e., 0.4 mM p-benzoquinone; pH 7.0 respiratory substrates solution) were measured, and 3,5-dichlorophenol (DCP), Ametryn, Acephate and Thiram were chosen as target toxicants. The selection basis for several pesticides is that we chose typical pesticides of different chemical structures. Ametryn, Acephate and Thiram are a triazine weedkiller, an organophosphorus insecticide and a sulfur fungicide respectively, which are widely used and were thus chosen as target toxicants. Inhibition percentage values are calculated according to eqn (1). Fig. 7 shows the respiration rates obtained at different concentrations of DCP and three pesticides, and the IC50 values of 9.83 mg L−1 for DCP, 22 mg L−1 for Ametryn, 29 mg L−1 for Acephate and 47.5 mg L−1 for Thiram were determined within 30 min. The lowest IC50 value corresponded to DCP, followed by Acephate, Ametryn and Thiram, indicating its high toxicity. Table 1 compares IC50 results of DCP by using the present biosensor and others under comparable conditions. It can be seen that this proposed bioassay is more sensitive than biosensor (eukaryote T. Cutaneum),28 Biolog (activated sludge),29 CellSense (Genetically Engineered Bacterium E. coli PMP 101)30 and Bioluminescence (Vibrio qinghaiensis sp. Q67 and BF-2/luc1 cell)30,31 biotoxicity assays, further demonstrating the potential applications of this novel and disposable S. cerevisiae-based sensor bioassay in determination of pesticide toxicities.
| Toxicants, IC50 (mg L−1) | Biotoxicity assays | Microorganism | Reference |
|---|---|---|---|
| 3,5-DCP | |||
| 9.83 | Biosensor, amperometry | S. cerevisiae (pretreated) | This work |
| 32.1 | Whole cell biosensor, amperometry | T. Cutaneum (eukaryote) | 28 |
| 14.4 | Biolog MT2 microplates procedure, incubation 24 h | Activated sludge | 29 |
| 15.10 | CellSense, amperometry | Genetically engineered bacterium E. coli PMP 101 | 30 |
| 25.19 | Bioluminescence | Vibrio qinghaiensis sp. Q67 | 31 |
| 52.0 | Bioluminescence | BF-2/luc1 cell | 30 |
Furthermore, in order to demonstrate that IC50 values determined using the electrochemical method are reliable, three pesticides were investigated and the results compared with the published data for other methods. Table 2 reports the IC50 values for Ametryn, Acephate and Thiram as 22 mg L−1, 29 mg L−1 and 47.5 mg L−1, respectively. Obviously, Ametryn was the most toxic, Acephate was intermediate and Thiram was the least toxic of the three pesticides. Although the Toxalert assay using V. fischeri shows a more sensitive response to Ametryn, it is not suitable for measurement of turbid solutions due to the decreased light intensity. In contrast, measurements by the electrochemical method are not disrupted by turbidity, even when measuring suspensions, which is an advantage, especially for wastewater samples.1 Thus, improvement of the biosensor's system is needed for further investigation. Nevertheless, the prepared disposable S. cerevisiae biofilm modified amperometric microbial sensor can effectively determine the biotoxicity of organic toxicants especially for DCP, and another advantage is that it can provide constant monitoring of the microbial activity, which makes it possible to use for the real-time monitoring of water quality and early warning of emergent pollution.
| Pesticides, IC50 | Biotoxicity assays | Reference | ||
|---|---|---|---|---|
| Ametryn | Acephate | Thiram | ||
| 22 | 29 | 47.5 | S. cerevisiae (pretreated); biosensor | This work |
| 18.6 mg L−1 | — | — | V. fischeri; Toxalert, 30 min exposure | 32 |
| — | 141.25 ± 10.49 mM | — | Crassostrea hongkongensis; AChE activity | 33 |
| — | — | >40 mg L−1 (did not give the specific values) | M. phaseolina (eukaryote); in vitro | 34 |
3.5. Reproducibility and stability of the disposable microbial biosensor
The reproducibility of the prepared microbial biosensors was evaluated by five replicate assays for 10 mg L−1 DCP, Ametryn, Acephate and Thiram inhibition on S. cerevisiae. As shown in Fig. S4(a),† the relative standard deviations (RSD) obtained were 2.15%, 3.33%, 3.61% and 3.53%, respectively. Thus, the prepared biosensors showed a good and reproducible performance.To maintain stability, the integrated microbial biosensors were kept at 4 °C. As shown in Fig. S5(b),† the responses to 10 mg L−1 DCP, Ametryn, Acephate and Thiram were measured every two days and the results showed a relative standard deviation (RSD) of 5.55%, 6.52%, 7.0% and 7.3%, respectively, for one week. The results indicated that the biocompatibility of hybrid material was good and the pretreated S. cerevisiae cells could be applied for biosensor fabrication.
3.6. Analysis of four real wastewater samples
In real environmental pollution, a wide variety of chemicals simultaneously exist in water. This prepared disposable biofilm-modified amperometric microbial sensor can provide a sensitive, rapid, convenient assessment of overall composite toxicity and is a promising approach for early risk warning of acute water toxicity. The usual real wastewaters containing organics, inorganics or both were our selection basis for wastewater, in order to reflect the broader spectrum responses to water samples by our prepared sensor. The wastewaters which were taken from a river around suburban farms of Beijing, a garbage-treatment plant, an electroplating factory and a laboratory were measured as the real samples. A 10 μL real sample was added to the 10 mL pH 7.0 respiratory substrates to obtain the volume ratio 1% (v/v) and its biotoxicity was measured with the prepared biosensor. As shown in Fig. S6,† the integrated biosensor was sensitive to the four kinds of real wastewater samples and the inhibitory percentage values were 30.75% for the river around suburban farms, 44.81% for garbage wastewater, 59.07% for laboratory wastewater and 64.57% for electroplating wastewater. Therefore, the toxicity can be ranked in a descending order: electroplating wastewater > laboratory wastewater > garbage-treatment wastewater > river water.4. Conclusions
A novel, disposable and sensitive whole cells amperometric biosensor based on permeabilized S. cerevisiae has been fabricated successfully for the biotoxicity assessment of pesticides in water and three kinds of real wastewater samples. Amperometric measurement suggested that the S. cerevisiae cells treated with ethanol caused similar “mild” permeabilization of the S. cerevisiae cell membrane, which resulted in the electrode being more sensitive to DCP than the non-treated one. In optimized assays, IC50 values of 9.83 mg L−1 for DCP, 22 mg L−1 for Ametryn, 29 mg L−1 for Acephate and 47.5 mg L−1 for Thiram were determined. These results obtained were better than those of other toxicity bioassays. Moreover, this prepared disposable biofilm-modified amperometric microbial sensor can effectively determine the biotoxicity of real wastewater samples, showing that the prepared biosensor can be easily applied to a practical mobile system for biotoxicity assays of chemicals and environmental water monitoring. Therefore, the present disposable biofilm electrochemical biosensors not only provides the advantages of avoiding complicated operation and expensive electrode materials, and increasing sensitivity of response, but also the potential fors expanding the technique to utilize S. cerevisiae with complementary toxicity responses, thereby allowing use of the biotoxicity assay in a wide range of applications.Acknowledgements
The authors appreciate the supports of the International Science & Technology Cooperation Program of China (no. 2013DFG50150), the Natural Foundation of Sciences of the People's Republic of China (no. 21175144) and the Key Project of Beijing Natural Science Foundation (no. 2120002).Notes and references
- D. M. Yong, C. Liu, D. B. Yu and S. J. Dong, Talanta, 2011, 84, 7–12 CrossRef CAS PubMed.
- Y. V. Nancharaiah, M. Rajadirai and V. P. Venugopalan, Environ. Sci. Technol., 2007, 41, 2617–2621 CrossRef CAS.
- A. Tizzard, J. Webber, R. Gooneratne, R. John, J. Hay and N. Pasco, Anal. Chim. Acta, 2004, 522, 197–205 CrossRef CAS PubMed.
- J. L. Levy, J. L. Stauber and D. F. Jolley, Sci. Total Environ., 2007, 387, 141–154 CrossRef CAS PubMed.
- B. Zhu, Z. F. Wu, J. L. Fang and G. X. Wang, Ecotoxicol. Environ. Saf., 2011, 74, 2193–2202 CrossRef CAS PubMed.
- S. Belkin, Curr. Opin. Microbiol., 2003, 6, 206–212 CrossRef CAS.
- A. M. Horsburgh, D. P. Mardlin, N. L. Turner, R. Henkler, N. Strachan, L. A. Glover, G. I. Paton and K. Killham, Biosens. Bioelectron., 2002, 17, 495–501 CrossRef CAS.
- C. Y. Shao, C. J. Howe, A. J. R. Porter and L. A. Glover, Appl. Environ. Microbiol., 2002, 68, 5026–5033 CrossRef CAS.
- J. M. Parry, in Use of short- and medium-term tests for carcinogens and data on genetic effects in carcinogenic hazard evaluation, IARC Scientific Publications, Lyon, 1999, pp. 471–486 Search PubMed.
- R. M. Walmsley and P. Keenan, Biotechnol. Bioprocess Eng., 2000, 5, 387–394 CrossRef CAS.
- K. Esteve, C. Poupot, P. Dabert, M. Mietton-Peuchot and V. Milisic, J. Ind. Microbiol. Biotechnol., 2009, 36, 1529–1534 CrossRef CAS PubMed.
- L. Campanella, G. Favero, D. Mastrofini and M. Tomassetti, Sens. Actuators, B, 1997, 44, 279–285 CrossRef CAS.
- M. E. van der Rest, A. H. Kammings, A. Nakano, Y. Anraku, B. Poolman and W. N. Konings, Microbiol. Rev., 1995, 59, 304–322 CAS.
- R. Garjonyte, V. Melvydasb and A. Malinauskas, Bioelectrochemistry, 2008, 74, 188–194 CrossRef CAS PubMed.
- S. F. D'Souza, Biosens. Bioelectron., 2001, 16, 337–353 CrossRef.
- R. R. Chen, Appl. Microbiol. Biotechnol., 2007, 74, 730–738 CrossRef CAS PubMed.
- K. Catteralla, D. Robertson, S. Hudson, P. R. Teasdale, D. T. Welsh and R. John, Talanta, 2010, 82, 751–757 CrossRef PubMed.
- L. Liu, C. Y. Liu, L. Shang, D. Li, D. M. Yong, L. Qi and S. J. Dong, Talanta, 2010, 83, 31–35 CrossRef CAS PubMed.
- J. Y. Ma and J. M. Chen, Environ. Pollut., 2005, 136, 267–273 CrossRef CAS PubMed.
- P. S. Chen and C. S. Li, J. Environ. Monit., 2005, 7, 257–262 RSC.
- W. Laureyn, T. Stakenborg and P. Jacobs, in Handbook of Biosensors and Biochips, ed. R. Marks, D. Cullen, C. Lowe, H. H. Weetall, and I. Karube, Wiley, Chichester, 2007, pp. 1–19 Search PubMed.
- C. M. Hassan and N. A. Peppas, Adv. Polym. Sci., 2000, 153, 37–65 CrossRef CAS.
- Y. J. Wang, X. J. Yang, W. Tu and H. Y. Li, J. Microbiol. Methods, 2007, 68, 212–217 CrossRef CAS PubMed.
- T. Takei, K. Ikeda, H. Ijima and K. Kawakami, Process Biochem., 2011, 46, 566–571 CrossRef CAS PubMed.
- Y. J. Zhang and P. S. Cremer, Curr. Opin. Chem. Biol., 2006, 10, 658–663 CrossRef CAS PubMed.
- L. F. Dos-Santos, L. Defrenne and A. Krebs-Brown, Anal. Chim. Acta, 2002, 456, 41–54 CrossRef.
- J. S. Zhao, M. Wang, Z. Y. Yang, Z. Wang, H. S. Wang and Z. Y. Yang, Anal. Chim. Acta, 2007, 597, 67–74 CrossRef CAS PubMed.
- C. Liu, D. M. Yong, D. B. Yu and S. Dong, Talanta, 2011, 84, 766–770 CrossRef CAS PubMed.
- L. F. dos Santos, L. Defrenne and A. Krebs-Brown, Anal. Chim. Acta, 2002, 701, 41–54 CrossRef.
- A. Bentley, A. Atkinson, J. Jezek and D. M. Rawson, Toxicol. In Vitro, 2001, 5, 469–470 CrossRef.
- M. Ma, Z. Tong, Z. Wang and W. Zhu, Bull. Environ. Contam. Toxicol., 1999, 62, 247–254 CrossRef CAS.
- M. Farré, J. Fernandez, M. Paez, L. Granada, L. Barba, H. M. Gutierrez, C. Pulgarin and D. Barceló, Anal. Bioanal. Chem., 2002, 373, 704–709 CrossRef PubMed.
- G. C. Zha, V. P. Chen, W. K. W. Luk, X. G. Zou, R. C. Y. Choi and K. W. K. Tsim, Chem.-Biol. Interact., 2013, 203, 277–281 CrossRef CAS PubMed.
- R. F. B. Tonin, A. Avozani, A. L. D. Danelli, E. M. Reis, S. M. Zoldan and F. R. Garcés-Fiallos, Pesquisa Agropecuária Tropical, 2013, 43, 460–466 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra08468c |
| This journal is © The Royal Society of Chemistry 2014 |