Zinc oxide microrods and nanorods: different antibacterial activity and their mode of action against Gram-positive bacteria†
Abstract
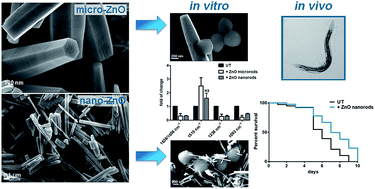
The development of antibiotic resistance among pathogenic bacteria combined with increased implant-associated infections have determined a great interest towards new bactericidal materials containing various organic and inorganic substances. Among them, zinc oxide (ZnO) derived materials have received considerable attention due to their unique antibacterial, antifungal, and UV filtering properties as well as high catalytic and photochemical activities. In the present work, we investigate the antimicrobial properties against two Gram-positive bacteria (Staphylococcus aureus and Bacillus subtilis) of ZnO microrods (MRs) and nanorods (NRs), produced in bulk quantities through simple and inexpensive methods. We demonstrate that the antimicrobial effect is strongly dependent on the rod size and dose. Scanning electron microscopy analysis revealed that the two investigated microbial types interact differently with the ZnO-MRs and NRs due to their different morphology. This resulted in different outcomes as reported by their respective Colony Forming Unit (CFU) capabilities. Moreover, Fourier Transform Infrared (FT-IR) spectroscopy revealed that changes in cell outer structures, i.e. membrane and exopolysaccharides (EPS), produced by the interaction with the ZnO structures, are responsible for the antimicrobial mechanism without the accumulation of reactive oxygen species. This was further strengthened by the increased survival observed in the case of bacterial cells treated in the presence of an osmotic support, like glycerol. In addition, Inductively Coupled Plasma Mass Spectrometry (ICP-MS) analysis showed that reduced cell viability is not strictly correlated to increased zinc ion release in the suspension. We then concluded that ZnO-NRs have a superior antimicrobial effect against both S. aureus and B. subtilis at much lower doses when compared to ZnO-MRs. This is mainly due to the smaller diameter of the NRs, which promotes surface damaging and protein alteration of the cell wall. Finally, the lack of toxicity and the antimicrobial properties of ZnO-NRs versus S. aureus, validated in vivo using the nematode Caenorhabditis elegans as host infection model, confirm the promising exploitation of ZnO-NRs in biomedical applications.

 Please wait while we load your content...
Please wait while we load your content...