Analysis of benzoylurea insecticides in water samples with TiO2 nanotube array micro-solid phase extraction coupled to high performance liquid chromatography
Abstract
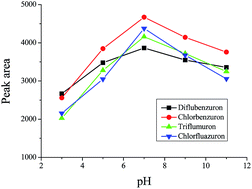
A rapid, easy to operate, and sensitive method was developed for the trace determination of benzoylurea insecticides based on a micro-solid phase extraction with highly ordered TiO2 nanotube arrays as the adsorbent prior to high performance liquid chromatography. The experimental results indicated that a good linear relationship was achieved between the peak areas and the concentrations of benzoylurea insecticides in the range of 0.1–40 μg L−1. The limits of detection (LODs) of diflubenzuron, chlorbenzuron, triflumuron and chlorfluazuron were 0.082, 0.026, 0.049 and 0.076 μg L−1, respectively. The proposed method was successfully used to determine the benzoylurea insecticides in environmental water samples. The spiked recoveries in the range of 76.5–102.4% were achieved. All these results demonstrated that the proposed method was of great value and would have great potential in the enrichment and determination of trace pollutants in the future.

 Please wait while we load your content...
Please wait while we load your content...