Antioxidant and hepatoprotective effects of an organic grapevine leaf (Vitis labrusca L.) extract in diabetic rats
Denise dos Santos Lacerdaa,
Carolina Ferreira Santosb,
Alice Spiecker Oliveirac,
Rafaela Zimmermannc,
Ricardo Schneider Jr.b,
Fabiana Agostinid,
Caroline Danicd,
Cláudia Funchalc and
Rosane Gomez*ab
aPrograma de Pós-Graduação em Ciência Biológicas: Fisiologia, Universidade Federal do Rio Grande do Sul (UFRGS), 90050-170, Porto Alegre, Brasil
bDepartamento de Farmacologia – Instituto de Ciências Básicas da Saúde, Universidade Federal do Rio Grande do Sul (UFRGS), Rua Sarmento Leite, 500/202, Porto Alegre, RS 90050-170, Brasil. E-mail: rosane.gomez@ufrgs.br
cCentro Universitário Metodista do IPA, 90420-060, Porto Alegre, Brasil
dUniversidade de Caxias do Sul, UCS, 95070-560, Caxias do Sul, Brasil
First published on 14th October 2014
Abstract
Our objective was to investigate the antioxidant effect of an aqueous extract of organic grapevine leaves (Vitis labrusca L.) on the livers of diabetic rats and to evaluate the resulting changes in metabolic and biochemical parameters. Diabetic rats received daily intragastric doses of 50, 100 or 200 mg kg−1 of the grapevine extract for 30 days. Grapevine leaf extract showed a dose-dependent antioxidant effect on the livers of diabetic rats, evidenced by decreases in TBARS and in carbonyl levels and increases in sulfhydryl levels. Moreover, the extract (200 mg kg−1) prevented weight loss and reduced LDL cholesterol (50 mg kg−1), urea (50 mg kg−1), and AST (50 and 100 mg kg−1) levels in diabetic rats at the indicated doses. Thus, we suggest that chronic treatment with an extract of grapevine leaves may represent an adjuvant therapy for the treatment and/or prevention of diabetic complications because of its antioxidant, hepatoprotective and possible hypolipidemic effects shown here.
Introduction
Diabetes mellitus (DM), characterized by a deficiency in the secretion and/or action of insulin, causes chronic hyperglycemia that results in metabolic and oxidative imbalances.1 Studies have shown that chronic hyperglycemia activates different cellular pathways, promoting the auto-oxidation of glucose, lipid peroxidation, changes in the activities of antioxidant enzymes, and the alteration of glutathione metabolism. All of these mechanisms are associated with oxidative stress and tissue damage and, consequently, with the development of diabetic complications.2–4Diabetes is a well-known disease with many therapeutic options for controlling hyperglycemia. Among these options, herbal treatments have gained special attention as agents to prevent and treat diabetic complications.5 Herbal medicines, such as grapevine and its byproducts, are rich in phytochemicals that possess antioxidant properties.6–8 These antioxidants present in vines and their byproducts modulate metabolism and improve the endogenous antioxidant system, decreasing the cellular damage caused by reactive species.9
Phenolic compounds and other bioactive phytochemicals are diversely distributed in different parts of the grapevine plant, such as the stem, leaves, seeds, and fruit, and they change according to the environment challenges inherent in organic viticulture.9–11 Organic viticulture is characterized by restrictions against the use of synthetic pesticides and fertilizers, a practice of farming that can produce differences in phenolic content compared to conventional culture methods.6,12 Indeed, Dani and colleagues (2010)7 showed higher values of total polyphenols and resveratrol antioxidant activity in grapevines cultivated by organic methods than in those cultivated conventionally.
Although the antioxidant properties of vines and grapevines have been very well characterized, few studies have focused on the leaves.13,14 As the leaves are discarded during vine production, their use for their therapeutic properties may represent an economic gain for farmers and an ecological gain for sustainable viticulture. Interestingly, red vine leaves from Vitis vinifera L. have been used as a food or food additive, particularly in Greek and Turkish cultures.9,15 Moreover, leaf extracts are used in traditional herbal medicine for the treatment of diarrhea and chronic venous insufficiency.15 Clinical studies also showed the efficacy of an aqueous red vine leaf extract in reducing edema and in improving microcirculation in humans.16 However, no studies have been performed to evaluate the possible therapeutic effects of organic grapevine leaves in diabetic individuals. Thus, our objective was to investigate the antioxidant effect of an aqueous extract of organic grapevine leaves (Vitis labrusca L.) on the livers of diabetic rats. Additionally, we explored the effects of this extract on different metabolic and biochemical parameters.
Material and methods
Animals
Male Wistar adult rats (270–300 g) from the Centro de Reprodução de Animais de Laboratório (CREAL) of the Universidade Federal do Rio Grande do Sul (UFRGS) were housed in polypropylene cages (40 × 33 × 17 cm), 3 or 4 per cage, under standard environmental conditions (room temperature, 22 ± 2 °C; 12 h light–dark cycle, 7 a.m.–7 p.m.). All rats had free access to food and water, except on the last day, during which they were fasted for 12 h prior to euthanasia and blood collection for biochemical analyses. Our experimental protocol was carried out in accordance with the International Guidelines for Use and Care of Laboratory Animals and with Brazilian laws for the Scientific Use of Animals. The protocol began after it had been approved by the Ethical Committee for Animal Experimentation at UFRGS (CEUA-UFRGS # 22445). All efforts were made to minimize animal suffering and to use the minimum number of animals necessary to produce reliable data.Grapevine leaves extract and chemicals
Grapevine leaves from Vitis labrusca var. Bordo (L.) were collected in November 2012 from an organic farm in Flores da Cunha City, RS, Brazil, and they were identified by Amaury Junior Silva, a botanist from the Herbarium of the Centro Universitário Metodista do IPA, Porto Alegre, RS, Brazil. The leaves were dried under shady conditions and coarsely powdered for extraction. Later, the powdered fresh leaves were weighed and placed in a flask containing distilled water at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (plant
10 (plant![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) solvent), and the extraction was performed in a closed reflux system (70 °C) for 1 h.6 A water bath (60 °C) was used to remove the solvent, yielding the dry crude extract. Next, the crude extract was reconstituted in saline solution to concentrations of 50, 100 and 200 mg mL−1. Streptozotocin (Sigma-Aldrich, St. Louis, USA) was prepared in citrate buffer (pH 4.5) immediately before its administration (60 mg kg−1 mL−1, i.p.). All other commercial reagents used were of analytical grade and were purchased from local suppliers.
solvent), and the extraction was performed in a closed reflux system (70 °C) for 1 h.6 A water bath (60 °C) was used to remove the solvent, yielding the dry crude extract. Next, the crude extract was reconstituted in saline solution to concentrations of 50, 100 and 200 mg mL−1. Streptozotocin (Sigma-Aldrich, St. Louis, USA) was prepared in citrate buffer (pH 4.5) immediately before its administration (60 mg kg−1 mL−1, i.p.). All other commercial reagents used were of analytical grade and were purchased from local suppliers.
Experimental groups and procedures
Half of the rats (n = 40) were rendered diabetic by a single dose of streptozotocin (60 mg kg−1) administered intraperitoneally (i.p.). Control rats (n = 40) received an injection of vehicle solution (1 mL kg−1, i.p.). Diabetes was confirmed 48 h later using a glucometer (Glucotrend, Boehringer Institute, Mannheim, Germany), and animals that had blood glucose levels lower than 200 mg dL−1 were discarded. Diabetic rats (STZ) were then randomly selected to receive daily doses (via oral gavage) of 50, 100, or 200 mg kg−1 of grapevine leaf extract or saline (n = 10 per group) for 30 days. Non-diabetic control rats (CTR) were subsequently allocated to receive saline or different doses of grapevine leaf extract following the same protocol used for the STZ rats.Grapevine leaf extract or saline treatment started upon 72 hours after streptozotocin administration. Daily gavage was performed in the morning (∼9 a.m.) for 30 days. On the 30th day, animals were euthanized by decapitation 30 min after administration of the extract and after a 12 h fast. The trunk blood was collected, centrifuged (1000 × g, 5 min) under refrigeration (5 °C), and used for the subsequent determination of biochemical parameters. The livers were dissected and weighed and then immediately frozen in liquid nitrogen and stored in a biofreezer (−80 °C) for subsequent measurements of oxidative stress parameters. Abdominal and epididymal fat was also dissected and weighed. The liver and fat weights were normalized to 100 g of body weight. Food and water intake were recorded every other day, and the body weight and blood glucose levels were measured weekly throughout the experiment.
Phytochemicals
An aliquot of the crude grapevine leaf extract was subjected to an analysis of the phenolic content. Total phenolic content was measured using Singleton and Rossi's modification of the Folin–Ciocalteu colorimetric method.17 The results were expressed as mg of gallic acid per mL. Flavonoid content was evaluated by the vanillin assay according to Monagas and colleagues18 (2003). Briefly, 2.5 mL of H2SO4–methanol (25/75, v/v) solution and 2.5 mL of 1% (w/v) vanillin dissolved in methanol were added to 1 mL of the aliquot. A blank that contained methanol without vanillin was prepared. The absorbance (500 nm) was read after 15 min at 30 °C, and the results are expressed as μg rutin per mL. Catechin, epicatechin, naringin, and resveratrol concentrations were evaluated by High Performance/Pressure Liquid Chromatography (HPLC) using an HP 1100 system equipped with a UV detector, a 5 μm LiChrospher® RP-18 column and a quaternary pump system using a 1.0 mL min−1 flow rate and a 50 μL injection volume. Catechin quantification was performed as described by Saucier and collegues19 (2001). Solvent A was water, and solvent B was methanol (both solvents contained 5 acetic acid); isocratic elution was performed using 90% solvent A and 10% solvent B. The total time of analysis (absorbance at 280 nm) was 45 min. For naringin quantification, solvent A was water, and solvent B was methanol with 5% acetic acid–acetonitrile (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15). A gradient elution was performed beginning with 99% solvent A and 1% of solvent B and ending with 100% of solvent B; the total time of analysis (absorbance at 280 nm) was 60 min.20 Resveratrol quantification was carried out as described by Ector and collegues21 (1996). For this measurement, solvent A was water at pH 2.5, and solvent B was methanol. A gradient elution was performed beginning with 90% solvent A and 10% solvent B and ending with 100% of solvent B; the total time of analysis (absorbance at 313 nm) was 45 min.21
15). A gradient elution was performed beginning with 99% solvent A and 1% of solvent B and ending with 100% of solvent B; the total time of analysis (absorbance at 280 nm) was 60 min.20 Resveratrol quantification was carried out as described by Ector and collegues21 (1996). For this measurement, solvent A was water at pH 2.5, and solvent B was methanol. A gradient elution was performed beginning with 90% solvent A and 10% solvent B and ending with 100% of solvent B; the total time of analysis (absorbance at 313 nm) was 45 min.21
Oxidative stress parameters
Samples from livers were obtained at ambient temperature, weighed, and homogenized for 30 s in 1.15% KCl buffer (5 mL per g tissue) and phenyl methyl sulfonyl fluoride (PMSF), a protease inhibitor, using an Ultra Stirrer (Model Ultra 80) according to Funchal and colleagues22 (2010). The homogenates were centrifuged (1000 × g, 10 min, at 5 °C; Hettich Universal 320R, London, UK). The supernatant was collected for subsequent determination of oxidative stress parameters. The protein concentrations in the samples were determined by the Lowry method using bovine serum albumin as the standard.23Lipid oxidative damage was determined in the liver homogenates by the thiobarbituric acid reactive substances (TBARS) method as described by Ohkawa and collegues24 (1979). Briefly, 50 μL of 8.1% sodium dodecyl sulfate (SDS), 375 μL of 20% acetic acid (pH 3.5), and 375 μL of 0.8% thiobarbituric acid (TBA) were added to 200 mL of homogenate and incubated in a boiling water bath for 60 min. Then, the supernatant was removed, and the absorbance (535 nm) was measured using a spectrophotometer (T80 UV/VIS Spectrometer, PG Instruments, Sao Paulo, BR). Malondialdehyde was used as a standard, and the results are expressed as nmol per mg protein.
Protein oxidative damage in liver samples was determined by the carbonyl assay according to Reznick and Packer25 (1994). The homogenates were incubated with 2,4 dinitrophenylhydrazine (DNPH 10 mmol L−1) in 2.5 mol L−1 HCl solution for 1 h in the dark at room temperature, with shaking every 15 min. Then, a 20% TCA (w/v) solution was added to the samples, which were placed on ice for 10 min and centrifuged (1000 × g for 5 min) to collect protein precipitates. Another wash was performed with 10% TCA, and the resulting pellet was washed three times with ethanol–ethyl acetate (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (v/v). Thereafter, the pellet was dissolved in 6 mol L−1 guanidine hydrochloride solution and incubated for 10 min at 37 °C, after which the absorbance (360 nm) was determined (T80 UV/VIS Spectrometer, PG Instruments, Sao Paulo, BR). The results are expressed as nmol per mg protein.
1) (v/v). Thereafter, the pellet was dissolved in 6 mol L−1 guanidine hydrochloride solution and incubated for 10 min at 37 °C, after which the absorbance (360 nm) was determined (T80 UV/VIS Spectrometer, PG Instruments, Sao Paulo, BR). The results are expressed as nmol per mg protein.
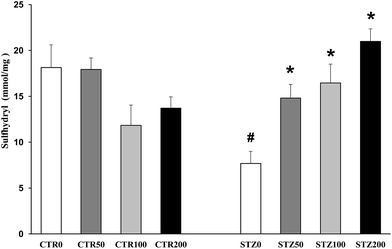
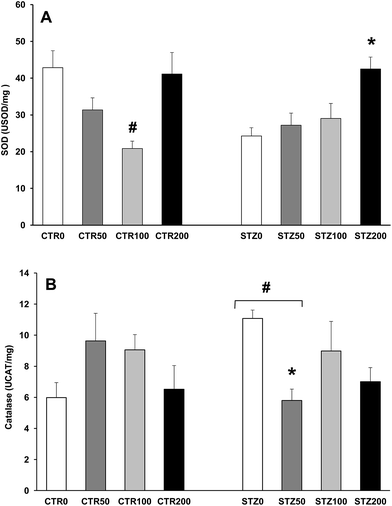
The sulfhydryl content, which represents a non-enzymatic antioxidant defense, is inversely correlated with oxidative protein damage. For the sulfhydryl assay, we added 0.1 mM DTNB to 120 μL of the liver samples and incubated them for 30 min at ambient temperature in a dark room as described by Aksenov and Markesberry26 (2001). Absorption was measured at 412 nm (T80 UV/VIS Spectrometer, PG Instruments), and the results are expressed as nmol per mg protein. Enzymatic defenses were evaluated by the measurement of superoxide dismutase (SOD) and catalase (CAT) activities. SOD activity, expressed as USOD per mg protein, was determined by the inhibition of the ratio of autocatalytic adrenochrome formation and read at 480 nm (T80 UV/VIS Spectrometer, PG Instruments.27 Moreover, CAT activity, expressed as UCAT per mg protein, was determined by the decrease in the absorption of hydrogen peroxide (H2O2) at 240 nm.28
Biochemical analysis
Levels of fasting glucose, triglycerides, total cholesterol, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) in serum samples were determined using commercial test kits (LABTEST, São Paulo, SP, Brazil) employing an enzymatic colorimetric method performed by automated equipment (CT 600 I, Labimbráz, Buenos Aires, Argentina). LDL-C and VLDL-C values were calculated by the Friedewald formula.29 The measurement of weekly blood glucose and glucose levels 48 h after streptozotocin administration were performed using a glucometer (Boehringer, Mannheim, Germany) by taking a blood drop from a superficial puncture at the distal end of the tail immediately before extract administration.Statistical analysis
The normality of the results was evaluated by the Shapiro–Wilks test. Parametric results were analyzed by 2-way ANOVA, with diabetes (STZ × CTR) and treatment with different concentrations of extract (50, 100, or 200 mg kg−1) as independent variables, followed by the Bonferroni test to detect differences between groups. Non-parametric results were analyzed by the Kruskal–Wallis test followed by Dunn's test. To compare the weekly consumption of food and water as well as non-fasting glycemia, we used a two-way repeated measures ANOVA. Differences were considered significant when P < 0.05. The results are expressed as the mean ± S.E.M. The data were analyzed using the Sigma Stat Program (Jandel Scientific Co., v. 11.0, San Jose, USA).Results
Phytochemicals in the grapevine leaf extract
Phytochemical analysis of our aqueous extract from organic grapevines leaves (Vitis vinifera L.) showed that catechin was the most prevalent flavonoid, followed by epicatechin and naringin (Table 1). Although resveratrol was detected, it was present at a very low concentration.| Total phenolic content (mg gallic acid per mL) | Total flavonoids (μg rutin per mL) | Naringin (mg per g raw extract) | Catechin (mg per g raw extract) | Epicatechin (mg per g raw extract) | Resveratrol (μg mL−1) | |
|---|---|---|---|---|---|---|
| a Values are presented as the mean ± standard deviation. | ||||||
| Organic grapevine leaf extract | 4.38 ± 9.01 | 26.08 ± 0.50 | 0.17 ± 0.01 | 5.73 ± 0.07 | 4.60 ± 0.10 | 0.003 ± 0.001 |
Oxidative parameters
Chronic hyperglycemia increased TBARS (P < 0.001; Fig. 1A), increased protein carbonyls (P = 0.012; Fig. 1B), and decreased free sulfhydryl levels (P < 0.001; Fig. 2) in the livers of STZ rats. However, the activities of antioxidant enzymes in the livers were either not affected (SOD: P > 0.05; Fig. 3A) or increased (CAT: P = 0.03; Fig. 3B) in the diabetic rats.The aqueous extract of organic grapevine leaves decreased lipid and protein damage in the liver of diabetic rats after 30 days of daily oral administration. These effects were dose dependent, with all doses decreasing TBARS (Fig. 1A) and increasing sulfhydryl levels (Fig. 2), with only the higher doses (100 and 200 mg kg−1) decreasing carbonyl levels in STZ rats (P < 0.05; Fig. 1C).
Grapevine leaf extract also increased SOD activity at doses of 200 mg kg−1 in the liver of the STZ rats (P = 0.015; Fig. 3A). Curiously, SOD activity decreased at doses of 100 mg kg−1 in the CTR rats (P < 0.001; Fig. 3A), and CAT activity decreased at doses of 50 mg kg−1 in the liver of the STZ rats (P = 0.014; Fig. 3B).
Metabolic parameters
Food and water intake were higher in the STZ rats than in the CTR rats (food intake: CTR0 = 22.4 ± 1.8 vs. STZ0 = 34.2 ± 1.6 g per rat per day, P = 0.001; water intake: CTR0 = 31.8 ± 3.7 vs. STZ0 = 116.3 ± 18.3 mL per rat per day, P = 0.003), and chronic treatment with the grapevine leaf extract did not affect these parameters.Moreover, as expected, the STZ rats lost weight (STZ0 = −70.0 ± 22.0 g), while the CTR rats gained weight (CTR0 = +33.2 ± 15.8 g) (P < 0.001), during the experimental period. However, the STZ rats treated with grapevine leaf extract at doses of 200 mg kg−1 (STZ200) lost approximately 40% less weight than the STZ0 rats (P < 0.001; Table 2). Accordingly, total fat (abdominal + epididymal fat) was 9.6 times higher in the STZ200 rats than in the STZ0 rats (P < 0.001), with a significant contribution from epididymal fat (P < 0.001) (Table 2). As shown in Table 2, the livers were heavier, and the AST and ALT levels were higher in the STZ rats compared with the CTR rats (P < 0.001). Grapevine leaf extract decreased AST levels at the 50 and 100 mg kg−1 dose but did not change any of the other parameter in diabetic rats.
| Liver weight (g) | Abdominal fat (g) | Epididymal fat (g) | AST (U L−1) | ALT (U L−1) | |
|---|---|---|---|---|---|
| a Values are presented as the mean ± standard deviation, except for AST, for which values are presented as the median [interquartile range]. n = 10 per group.b Different from the CTR groups.c Different from the STZ groups. | |||||
| CTR0 | 2.97 ± 0.57 | 1.33 ± 0.52 | 1.16 ± 0.25 | 145 [32] | 66.2 ± 29.9 |
| CTR50 | 3.00 ± 0.65 | 1.37 ± 0.38 | 1.26 ± 0.29 | 129 [67] | 57.0 ± 13.4 |
| CTR100 | 3.02 ± 0.57 | 1.48 ± 0.35 | 1.38 ± 0.14 | 86 [78] | 43.0 ± 21.6 |
| CTR200 | 3.04 ± 0.69 | 1.44 ± 0.22 | 1.48 ± 0.24c | 115 [99] | 44.6 ± 21.9 |
| STZ0 | 3.63 ± 0.36b | 0.00 ± 0.02b | 0.03 ± 0.07b | 256 [68]b | 203.1 ± 26b |
| STZ50 | 3.66 ± 0.46b | 0.00 ± 0.06b | 0.01 ± 0.02b | 98 [87]b,c | 115.6 ± 48.9b |
| STZ100 | 3.52 ± 0.24b | 0.01 ± 0.04b | 0.07 ± 0.16b | 137 [98]b,c | 94.4 ± 66.5b |
| STZ200 | 3.58 ± 0.23b | 0.05 ± 0.07b | 0.28 ± 0.20b,c | 418 [212]b | 242.2 ± 206.0b |
| P | <0.001 | <0.001 | <0.05 | <0.05 | <0.001 |
Adding to these biochemical parameters, we also showed that diabetes increased fasting glycemia, triglycerides, LDL-, and VLDL-cholesterol (P < 0.05; Table 3). The only parameter that was decreased by chronic treatment with grapevine leaf extract was LDL-cholesterol at the 50 mg kg−1 dose. Non-fasting weekly glycemia was also much higher in the STZ rats than in the CTR rats, and treatment with the extract did not affect that difference.
| Glucose (mg dL−1) | Triglycerides (mg dL−1) | Total cholesterol (mg dL−1) | HDL-C (mg dL−1) | LDL-C (mg dL−1) | VLDL (mg dL−1) | |
|---|---|---|---|---|---|---|
| a Values are presented as the mean ± standard deviation, n = 10 per group; 2-way ANOVA + Bonferroni test.b Different from CTR.c Different from CTR0.d Different from STZ0. | ||||||
| CTR0 | 111.8 ± 9.8 | 55.8 ± 26.2 | 39.0 ± 11.1 | 16.2 ± 4.8 | 11.6 ± 3.1 | 11.1 ± 5.2 |
| CTR50 | 97.4 ± 29.4 | 51.5 ± 67.5 | 34.4 ± 6.3 | 16.5 ± 4.2 | 8.8 ± 5.7c | 16.0 ± 13.5 |
| CTR100 | 93.6 ± 40.3 | 39.8 ± 25.9 | 30.2 ± 13.9 | 15.7 ± 7.2 | 9.4 ± 4.3 | 7.9 ± 5.2 |
| CTR200 | 97.0 ± 34.3 | 35.4 ± 24.9 | 33.6 ± 9.8 | 14.8 ± 4.9 | 11.7 ± 4.7 | 7.0 ± 4.9 |
| STZ0 | 503.4 ± 101.5b | 72.8 ± 47.8b | 42.4 ± 12.2 | 19.8 ± 5.9b | 12.5 ± 8.6 | 10.0 ± 4.3b |
| STZ50 | 460.2 ± 81.0b | 150.8 ± 109.3b | 36.6 ± 14.9 | 14.8 ± 3.7b | 3.7 ± 7.2d | 30.1 ± 21.9b |
| STZ100 | 455.6 ± 140.2b | 70.2 ± 47.9b | 41.2 ± 18.2 | 19.0 ± 7.9b | 8.1 ± 6.7 | 14.0 ± 9.6b |
| STZ200 | 462.8 ± 124.4b | 101.0 ± 43.2b | 51 ± 15.3 | 26.0 ± 5.9b | 11.6 ± 3.1 | 5.3 ± 8.9b |
| P | <0.001 | <0.050 | 0.071 | <0.050 | 0.009 | 0.0380 |
Discussion
Natural products represent a therapeutic alternative for the treatment and/or prevention of diabetic complications. The beneficial effects of vines or their byproducts on human health have been established.30 Indeed, polyphenols and other antioxidant compounds in vines protect against the cellular damage caused by chronic hyperglycemia and against other metabolic disturbances in diabetic individuals. In the present study, we showed the antioxidant effect of an aqueous extract of organic grapevine leaves in the livers of diabetic rats. Additionally, we found dose-dependent hepatoprotective and possible hypolipidemic effects of grapevine leaf extract.The biosynthesis of polyphenols and other antioxidants occurs in all parts of the plant at different levels in response to environmental conditions. Leaves are discarded from viticulture in most countries because they are not of commercial interest. However, studies have shown that leaves exhibit antioxidant properties, especially when they are harvested using organic methods.7,9,10 Additionally, the method of extraction may increase the concentration of antioxidants in leaf extracts. Indeed, we showed that aqueous extraction of organic grapevine leaves yielded a 3-fold greater flavonoid concentration than did ethanolic extraction (Table 1).7 We found also twice polyphenols levels in our extract than an aqueous extract from conventionally-produced grapevine leaves.13
In diabetic individuals, chronic hyperglycemia activates different extra- and intra-cellular biochemical cascades, promoting tissue damage in multiple organs.1–3 Recapitulating the results of others, we showed here that diabetic rats exhibited increased TBARS and carbonyl levels (Fig. 1) and decreased sulfhydryl (Fig. 2) content in their livers, reflecting lipid and protein oxidative damage to this tissue.2,13 Studies suggest that the auto-oxidation of free glucose and the consequent production of reactive species are partially responsible for the lipid and protein damage observed in the liver of diabetic individuals.2 Disturbances in metabolism also contribute to hepatic damage, as diabetes increases hepatic lipogenesis, enhanced free fatty acids, and the production of reactive oxygen species by mitochondria.2
Here we showed that chronic treatment with organic grapevine leaf extract significantly decreased lipid peroxidation and carbonyl levels and increased sulfhydryl content in the livers of diabetic rats, revealing the antioxidant and hepatoprotective properties of organic grapevine leaf extract (Fig. 1). Other studies also highlight the hepatoprotective effect of grapevine extracts against lipid and protein damage in rodents.14,31,32 These effects have been attributed to the antioxidant properties of the plant. Flavonoids, for example, present particular structural and conformational characteristics, such as a phenol group, that effectively scavenge free radicals.33 Flavonoids may also act by specific mechanisms, such as chelating transition metals and interfering with enzyme activities or cell signaling pathways, thereby affecting gene expression and modulating the function of receptors, among other parameters.8,30 Indeed, studies have shown that flavonoids, such as epicatechins and catechins, have a strong affinity for the lipid bilayer in addition to the ability to chelate transition metals, thereby decreasing protein oxidation and lipid peroxidation in different tissues.8,34 The catechol moiety of polyphenols also exhibits a high affinity for metal ions, thereby decreasing oxidative damage.30,35 Moreover, the improvement in the non-enzymatic antioxidant profile, as observed in our study by the increased sulfhydryl content, can be attributed, in part, to a stimulation of glutamylcysteine synthase activity by flavonoids. This enzyme is critical for the synthesis of glutathione, the most important non-enzymatic antioxidant defense, due to the presence of its thiol groups.30,36 All of these mechanisms may explain the antioxidant and hepatoprotective properties of our grapevine leaf extract.
By measuring antioxidant enzyme activities in liver tissue, we observed that diabetes did not affect SOD activity and, curiously, increased CAT activity (Fig. 3). The results showing the activities of antioxidant enzymes in the livers of diabetic animals point to no change or to a decrease.5 Although SOD activity was not changed by diabetes, the highest dose of the extract increased SOD activity in the STZ rats. These results are in accord with others that showed a hepatoprotective effect of treatment with grapevine juice and seed extract.31,37 Increases in SOD activity could be explained by an increased expression of transcription factors, such as Nrf2 (nuclear factor (erythroid-derived 2)-like 2), by grapevine leaf extract. Indeed, studies show an association among polyphenols, activation of Nrf2, and increased SOD expression.38,39 Additionally, we do not discard that increases on SOD at 200 mg kg−1 dose may be related to an endogenous response to a pro-oxidative mechanism of this specific dose. Ali et al.,40 2013, showed that the Mn porphyrin (MnPs), a synthetic SOD mimics that also scavenger superoxide at the site of mitochondrial electron transport chain show a pro- and antioxidant and it may either protect from or enhance the STZ-induced diabetic complication, depending on the timing and dose of antioxidant administration. Thus, enhanced SOD activity at 200 mg kg−1 dose may represent an adaptive response as a result of the pro-oxidative mechanism of action of this dose of the extract.
Although unexpected, the increased CAT activity in our STZ0 rats could be explained as by a physiological compensatory mechanism because this enzyme catalyzes the degradation of hydrogen peroxide, which is increased by chronic hyperglycemia in diabetic rats.41 Because the increased CAT activity was not matched by increased SOD activity, we hypothesize that CAT activity was activated by hydrogen peroxide from other fonts, such as mitochondria and/or cellular NADPH-dependent oxidases, as a response to chronic hyperglycemia.42 Grapevine leaf extract decreased CAT activity at doses of 50 mg kg−1 in diabetic animals, showing that antioxidants in the extract neutralized reactive species production.8,30 Thus, although not well explored, the leaves from grapevines also show antioxidant properties and may represent an alternative to preventing the cellular damage caused by chronic hyperglycemia in diabetic individuals.
Orhan and colleagues13 (2006) showed also higher lipoperoxidaton in the liver of diabetic than control rats, however, they did not find and antioxidant effect of grapevine leaves extract produced by conventional cultivation at doses of 250 mg kg−1, after 15 days of daily i.p. administration. For higher dose as 500 mg kg−1, they found only an increase on GSH activity at the end of this period. Discrepancies from our results could be attributed to treatment duration and via, since our rats were treated for 30 days by intragastric administration. Moreover, we presented a more complete antioxidant profile, since we considered here lipid and protein damage, beside enzymatic and non-enzymatic antioxidant parameters while those authors13 analyzed only MDA and GSH parameters.
In addition to the oxidative stress parameters described above, we explored the metabolic effects of the grapevine leaf extract. A dose-dependent effect of the extract on diabetes-induced hepatic damage was observed. Doses of 50 and 100 mg kg−1 of grapevine leaf extract decreased serum AST levels in diabetic animals, revealing the hepatoprotective effect of this extract (Table 2). The increase in hepatic AST and ALT enzyme levels in our diabetic animals could be related to structural and functional damage to hepatocytes as a result of increasing oxidative and inflammatory events as well as by augmenting ketogenesis and gluconeogenesis and by inducing an insulin deficit.43 Grapevine leaf extract also showed a hepatoprotective effect in alcoholic and cirrhotic rats, in which the extract treatment decreased AST levels.14,32 Because flavonoids exhibit antioxidant and anti-inflammatory properties, we infer that these protective mechanisms are related to the lowest AST levels observed after chronic treatment with our aqueous grapevine leaf extract.
Furthermore, we showed that the highest dose (200 mg kg−1) of grapevine leaf extract decreased the weight lost and preserved epididymal fat in these rats (Table 2). Although body weight gain is also observed upon the administration of other plant extracts to diabetic rats,44 we showed here, for the first time, that epididymal fat was mainly responsible for this effect. Indeed, Ghorbani and colleagues45 (2010) showed that type-1 diabetes induces a fat mass reduction in a depot-specific manner, with less fat mobilization from fat depots close to survival organs, such as epididymal fat depots. These effects may be related to the effects of flavonoids on lipases or on the modulation of cell signaling pathways.8
It is known that insulin deficiency changes lipid metabolism and increases free fatty acids and the synthesis of lipoproteins, causing dyslipidemia, which is related to atherosclerosis and the increased risk for cardiovascular diseases in diabetic individual.46 Our results showed that LDL-cholesterol decreased by the administration of the grapevine leaf extract at a dose of 50 mg kg−1, highlighting its potential hypolipidemic effect for both diabetic and non-diabetic rats (Table 3). Grapevine seed extract also reduces LDL-cholesterol in the livers of rats fed a high-fat diet.47 The hypolipidemic effects of grape polyphenols are attributed, at least in part, to a decrease in gastrointestinal fat absorption because rats fed with catechin dry matter show increased fat excretion in their feces.48 Moreover, polyphenols in grape seed also regulate the expression of genes responsible for controlling lipoprotein homeostasis in the livers of rats.49 Del Bas and colleagues49 (2005) showed that grape seed procyanidins increased cholesterol elimination via bile acids by inducing the overexpression of cholesterol 7alpha-hydroxylase (CYP7A1) and by increasing the expression of small heterodimer partner (SHP), a nuclear receptor, both of which are responsible for lipid homeostasis in the liver. Additionally, polyphenols decrease 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase activity in type 2 diabetic rats.50 This enzyme catalyzes the rate-limiting step of cholesterol synthesis in the liver, and its decreased activity would explain the decrease in LDL cholesterol observed in our STZ rats. Polyphenols also increase HMG-CoA lyase activity in vitro.51 This enzyme cleaves HMG-CoA to yield acetoacetate, and its inhibition prevents ketoacidosis, a common metabolic imbalance observed in diabetic individuals.51 Together, these mechanisms may explain the possible hypolipidemic effect exhibited by the grapevine leaf extract in our diabetic and non-diabetic rats. Moreover, in vitro studies point that red wine and resveratrol increases the expression and activity of the LDL-cholesterol receptor in human HepG2 cells.52,53 The up regulation of HDL receptors could also explain the lower LDL-cholesterol plasma levels in our rats. However, this effect was showed only for the lowest dose (50 mg kg−1). In pharmacology, such a result occurs frequently, and other natural products show a similar dose–response effect.54
In summary, we showed here that chronic treatment with an aqueous extract from organic grapevine leaves (Vitis labrusca L.) results in a dose-dependent antioxidant effect in the livers of diabetic rats. Additionally, we showed the hepatoprotective and possible hypolipidemic effects of grapevine leaf extract in addition to its effect on reducing body weight loss in diabetic rats. Thus, organic grapevine leaf extract, beyond other byproducts from the cultivation of vines, shows a beneficial effect and may represent an adjuvant therapy for the treatment and/or prevention of diabetic complications. As a residual product of viticulture, the use of leaves for this purpose would represent an economic advantage by promoting a sustainable agriculture.
Conflict of interest
The authors declare no conflict of interest.Acknowledgements
We would like to thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), PROPESQ – UFRGS, and Centro Universitário Metodista do IPA. The authors are grateful to fellowships from CNPq to DL, CS, RS, AO, and CF.References
- A. C. Maritim, R. A. Sanders and J. B. Watkins, Diabetes, Oxidative Stress, and Antioxidants, a Review, J. Biochem. Mol. Toxicol., 2003, 17, 24–38 CrossRef CAS PubMed.
- A. Dey and J. Lakshmanan, The role of antioxidants and other agents in alleviating hyperglycemia mediated oxidative stress and injury in liver, Food Funct., 2013, 4, 1148–1184 CAS.
- F. Giacco and M. Brownlee, Oxidative stress and diabetic complications, Circ. Res., 2010, 107, 1058–1070 CrossRef CAS PubMed.
- S. V. McLennan, S. Heffernan, L. Wright, C. Rae, E. Fisher, D. K. Yue and J. R. Turtle, Changes in hepatic glutathione metabolism in diabetes, Diabetes, 1991, 40, 344–348 CrossRef CAS.
- R. Singh, N. Kaur, L. Kishore and G. K. Gupta, Management of diabetic complications: a chemical constituent based approach, J. Ethnopharmacol., 2013, 150, 51–70 CrossRef CAS PubMed.
- C. Dani, L. S. Oliboni, R. Vanderlinde, D. Bonatto, M. Salvador and J. A. Henriques, Phenolic content and antioxidant activities of white and purple juices manufactured with organically- or conventionally-produced grapes, Food Chem. Toxicol., 2007, 45, 2574–2580 CrossRef CAS PubMed.
- C. Dani, L. S. Oliboni, F. Agostini, C. Funchal, L. Serafini, J. A. Henriques and M. Salvador, Phenolic content of grapevine leaves (Vitis labrusca var. Bordo) and its neuroprotective effect against peroxide damage, Toxicol. In Vitro, 2010, 24, 148–153 CrossRef CAS PubMed.
- C. G. Fraga, M. Galleano, S. V. Verstraeten and P. I. Oteiza, Basic biochemical mechanisms behind the health benefits of polyphenols, Mol. Aspects Med., 2010, 31, 435–445 CrossRef CAS PubMed.
- M. Monagas, B. Hernández-Ledesma, C. Gómez-Cordovés and B. Bartolomé, Commercial dietary ingredients from Vitis vinifera L. leaves and grape skins. Antioxidant and chemical characterization, J. Agric. Food Chem., 2006, 54, 319–327 CrossRef CAS PubMed.
- E. Schneider, H. Von der Heydt and A. Esperester, Evaluation of polyphenol composition in red leaves from different varieties of Vitis vinifera, Planta Med., 2008, 74, 565–752 CrossRef CAS PubMed.
- A. Teixeira, J. Eiras-Dias, S. D. Castellarin and H. Gerós, Berry phenolics of grapevine under challenging environments, Int. J. Mol. Sci., 2013, 14, 18711–18739 CrossRef CAS PubMed.
- D. K. Asami, Y. J. Hong, D. Barrett and A. E. Mitchell, Comparison of the total phenolic and ascorbic acid content of freeze-dried and air-dried marionberry, strawberry, and corn grown using conventional, organic, and sustainable agricultural practices, J. Agric. Food Chem., 2003, 51, 1237–1241 CrossRef CAS PubMed.
- N. Orhan, M. Aslan, D. D. Orhan, F. Ergun and E. Yeşilada, In vivo assessment of antidiabetic and antioxidant activities of grapevine leaves (Vitis vinifera) in diabetic rats, J. Ethnopharmacol., 2006, 108(2), 280–286 CrossRef CAS PubMed.
- L. Pari and A. Suresh, Effect of grape (Vitis vinefera L.) leaf extract on alcohol induced oxidative stress in rats, Food Chem. Toxicol., 2008, 46, 1627–1634 CrossRef CAS PubMed.
- M. Kosar, E. Küpeli, H. Malyer, V. Uylaser, C. Türkben and K. H. Baser, Effect of brining on biological activity of leaves of Vitis vinifera L. (Cv. Sultani Cekirdeksiz) from Turkey, J. Agric. Food Chem., 2007, 55, 4596–4603 CrossRef CAS PubMed.
- U. Kalus, J. Koscielny, A. Grigorov, E. Schaefer, H. Peil and H. Kiesewetter, Improvement of cutaneous microcirculation and oxygen supply in patients with chronic venous insufficiency by orally administered extract of red vine leaves AS 195, A randomised, double-blind, placebo-controlled, crossover study, Drugs R&D, 2004, 5, 63–71 CAS.
- V. L. Singleton, R. Orthofer and R. M. Lamuela-Raventos, Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent, in Methods in Enzymol, Oxidant and Antioxidant (Part A), ed. L. Packer, Academic Press, San Diego, 1999, vol. 299, pp. 159–178 Search PubMed.
- M. Monagas, V. Nunes, B. Bartolomé and C. Gómez-Cordovés, Anthocyanin derived pigments in Graciano, Tempramillo, and Cabernet Sauvignon wines produced in Spain, Am. J. Enol. Vitic., 2003, 54, 163–169 CAS.
- C. Saucier, M. Mirabel, F. Daviaud, A. Longieras and Y. Glories, Rapid fractionation of grape seed proanthocyanidins, J. Agric. Food Chem., 2001, 49, 5732–5735 CrossRef CAS PubMed.
- K. Robards, Strategies for the determination of bioactive phenols in plants, fruits and vegetables, J. Chromatogr. A, 2003, 1000, 657–691 CrossRef CAS.
- B. J. Ector, J. B. Magee, C. P. Hegwood and M. J. Coign, Resveratrol concentration in muscadine berries, juice, pomace, purees, seeds, and wines, Am. J. Enol. Vitic., 1996, 47, 57–62 CAS.
- C. Funchal, C. A. Carvalho, T. Gemelli, A. S. Centeno, R. B. Guerra, M. Salvador, C. Dani, A. Coitinho and R. Gomez, Effect of acute administration of 3-butyl-1-phenyl-2-(phenyltelluro)oct-en-1-one on oxidative stress in cerebral cortex, hippocampus, and cerebellum of rats, Cell. Mol. Neurobiol., 2010, 30, 1135–1142 CrossRef CAS PubMed.
- O. H. Lowry, N. J. Rosebrough, A. L. Farr and R. J. Randall, Protein measurement with the Folin phenol reagent, J. Biol. Chem., 1951, 193(1), 265–267 CAS.
- H. Ohkawa, H. Ohishi and K. Yagi, Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction, Anal. Biochem., 1979, 95, 351–358 CrossRef CAS.
- A. Z. Reznick and L. Packer, Oxidative damage to proteins: spectrophotometric method for carbonyl assay, Methods Enzymol., 1994, 233, 357–363 CAS.
- M. Y. Aksenov and W. R. Markesbery, Change in thiol content and expression of glutathione redox system gene in the hippocampus and cerebellum in Alzheimer's disease, Neurosci. Lett., 2001, 302, 141–145 CrossRef CAS.
- J. V. Bannister and L. Calabrese, Assays for superoxide dismutase, Methods Biochem. Anal., 1987, 32, 279–312 CAS.
- H. Aebi, Catalase in vitro, Methods Enzymol., 1984, 105, 121–126 CAS.
- A. J. Tremblay, H. Morrissette, J. M. Gagné, J. Bergeron, C. Gagné and P. Couture, Validation of the Friedewald formula for the determination of low-density lipoprotein cholesterol compared with beta-quantification in a large population, Clin. Biochem., 2004, 37, 785–790 CrossRef CAS PubMed.
- R. Rodrigo, A. Miranda and L. Vergara, Modulation of endogenous antioxidant system by wine polyphenols in human disease, Clin. Chim. Acta, 2011, 412, 410–424 CrossRef CAS PubMed.
- I. C. Chis, M. I. Ungureanu, A. Marton, R. Simedrea, A. Muresan, I. D. Postescu and N. Decea, Antioxidant effects of a grape seed extract in a rat model of diabetes mellitus, Diabetes Vasc. Dis. Res., 2009, 6, 200–204 CrossRef PubMed.
- D. D. Orhan, N. Orhan, E. Ergun and F. Ergum, Hepatoprotective effect of Vitis vinifera L. leaves on carbon tetrachloride-induced acute liver damage in rats, J. Ethnopharmacol., 2007, 112, 145–151 CrossRef PubMed.
- J. A. Ross and C. M. Kasum, Dietary flavonoids: bioavailability, metabolic effects, and safety, Annu. Rev. Nutr., 2002, 22, 19–34 CrossRef CAS PubMed.
- T. W. Sirk, E. F. Brown, M. Friedman and A. K. Sum, Molecular binding of catechins to biomembranes: relationship to biological activity, J. Agric. Food Chem., 2009, 57, 6720–6728 CrossRef CAS PubMed.
- N. R. Perron and J. L. Brumaghim, A review of the antioxidant mechanisms of polyphenol compounds related to iron binding, Cell Biochem. Biophys., 2009, 53, 75–100 CrossRef CAS PubMed.
- J. Ø. Moskaug, H. Carlsen, M. C. Myhrstad and R. Blomhoff, Polyphenols and glutathione synthesis regulation, Am. J. Clin. Nutr., 2005, 81, 277S–283S CAS.
- A. D. Rodrigues, T. B. Scheffel, G. Scola, M. T. Dos Santos, B. Fank, C. Dani, R. Vanderlinde, J. A. Henriques, A. S. Coitinho and M. Salvador, Purple grape juices prevent pentylenetetrazol-induced oxidative damage in the liver and serum of Wistar rats, Nutr. Res., 2013, 33, 120–125 CrossRef CAS PubMed.
- A. Murakami and K. Ohnishi, Target molecules of food phytochemicals: food science bound for the next dimension, Food Funct., 2012, 3, 462–476 CAS.
- P. Palsamy and S. Subramanian, Resveratrol protects diabetic kidney by attenuating hyperglycemia-mediated oxidative stress and renal inflammatory cytokines via Nrf2-Keap1 signaling, Biochim. Biophys. Acta, 2011, 1812, 719–731 CrossRef CAS PubMed.
- D. K. Ali, M. Oriowo, A. Tovmasyan, I. Batinic-Haberle and L. Benov, Late administration of Mn porphyrin-based SOD mimic enhances diabetic complications, Redox Biol., 2013, 1(1), 457–466 CrossRef CAS PubMed.
- S. Genet, R. K. Kale and N. Z. Baquer, Alteration in antioxidant enzymes and oxidative damage in experimental diabetic rat tissues, effect of vanadate and fenugreek (Trigonellafoenum graecum), Mol. Cell. Biochem., 2002, 236, 7–12 CrossRef CAS.
- W. M. Nauseef, Detection of superoxide anion and hydrogen peroxide production by cellular NADPH oxidases, Biochim. Biophys. Acta, 2014, 1840, 757–767 CrossRef CAS PubMed.
- A. Picardi, D. D'Avola, U. V. Gentilucci, G. Galati, E. Fiori, S. Spataro and A. Afeltra, Diabetes in chronic liver disease: from old concepts to new evidence, Diabetes/Metab. Res. Rev., 2006, 22, 274–283 CrossRef CAS PubMed.
- A. Kamboj, S. Kumar and V. Kumar, Evaluation of Antidiabetic Activity of Hydroalcoholic Extract of Cestrum nocturnum Leaves in Streptozotocin-Induced Diabetic Rats, Adv. Pharmacol. Sci., 2013, 2013, 1–4 CrossRef PubMed.
- A. Ghorbani, M. Varedi, M. A. Hadjzadeh and G. H. Omrani, Type-1 diabetes induces depot-specific alterations in adipocyte diameter and mass of adipose tissues in the rat, Exp. Clin. Endocrinol. Diabetes, 2010, 118, 442–448 CrossRef CAS PubMed.
- A. D. Mooradian, Dyslipidemia in type 2 diabetes mellitus, Nat. Clin. Pract. Endocrinol. Metab., 2009, 5, 150–159 CrossRef CAS PubMed.
- H. Quesada, J. M. del Bas, D. Pajuelo, S. Díaz, J. Fernandez-Larrea, M. Pinent, L. Arola, M. J. Salvadó and C. Bladé, Grape seed proanthocyanidins correct dyslipidemia associated with a high-fat diet in rats and repress genescontrolling lipogenesis and VLDL assembling in liver, Int. J. Obes., 2009, 33, 1007–1012 CrossRef CAS PubMed.
- L. Bravo, R. Abia, M. Eastwood and F. Saura-Calixto, Degradation of polyphenols (catechin and tannic acid) in the rat intestinal tract. Effect on colonic fermentation and faecal output, Br. J. Nutr., 1994, 71, 933–996 CrossRef CAS.
- J. M. Del Bas, J. Fernández-Larrea, M. Blay, A. Ardèvol, M. J. Salvadó, L. Arola and C. Bladé, Grape seed procyanidins improve atherosclerotic risk index and induce liver CYP7A1 and SHP expression in healthy rats, FASEB J., 2005, 19, 479–481 CAS.
- K. Shukla, P. Dikshit, M. K. Tyagi, R. Shukla and J. K. Gambhir, Ameliorative effect of Withania coagulans on dyslipidemia and oxidative stress in nicotinamide–streptozotocin induced diabetes mellitus, Food Chem. Toxicol., 2012, 50, 3595–3599 CrossRef CAS PubMed.
- S. Nakagawa, Y. Kojima, K. Sekino and S. Yamato, Effect of polyphenols on 3-hydroxy-3-methylglutaryl-coenzyme A lyase activity in human hepatoma HepG2 cell extracts, Biol. Pharm. Bull., 2013, 36, 1902–1906 CAS.
- S. Paul, C. Santos, P. Dubois, J. Mamo, K. Croft and E. Allister, Red wine polyphenolics increase LDL receptor expression and activity and suppress the secretion of ApoB100 from human HepG2 cells, J. Nutr., 2003, 133, 700–706 Search PubMed.
- T. Yashiro, M. Nanmouku, M. Shimizu, J. Inoue and R. Satos, Resveratrol increases the expression and activity of the low density lipoprotein receptor in hepatocytes by the proteolytic activation of the sterol regulatory element-binding proteins, Atherosclerosis, 2012, 220, 369–374 CrossRef CAS PubMed.
- M. Marounek, Z. Volek, D. Dušková, J. Tüma and T. Taubner, Dose–response efficacy and long-term effect of the hypocholesterolemic effect of octadecylpectinamide in rats, Carbohydr. Polym., 2013, 97, 772–775 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |