Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Vinylphenylglycidyl ether-based colloidal architectures: high-functionality crosslinking reagents, hybrid raspberry-type particles and smart hydrophobic surfaces†
S. Mehlhase‡
a,
C. G. Schäfer‡a,
J. Morsbachb,
L. Schmidta,
R. Kleina,
H. Frey*b and
M. Gallei*a
aErnst-Berl-Institut für Technische und Makromolekulare Chemie, Technische Universität Darmstadt, Alarich-Weiss-Str. 4, D-64287 Darmstadt, Germany. E-mail: m.gallei@mc.tu-darmstadt.de
bInstitute of Organic Chemistry, Organic and Macromolecular Chemistry, Johannes Gutenberg-Universität, Duesbergweg 10-14, D-55099 Mainz, Germany. E-mail: hfrey@uni-mainz.de
First published on 28th August 2014
Abstract
An efficient synthetic strategy for the preparation of monodisperse colloidal core/shell architectures with reactive epoxy functionalities is reported, based on the bifunctional vinylphenylglycidyl ether monomer. Accessibility of the reactive immobilized epoxy moieties was quantified by UV/vis spectroscopy in an isorefractive medium. Inorganic–organic hybrid raspberry-type architectures revealed a tremendous inpact on the surface wettability of water.
The design and control of hierarchical particle-based architectures play a key role in current polymer and colloid science in order to mimic sophisticated structures known from nature. Biomimetic strategies have created enormous potential for the development of multi-dimensional materials, featuring tailor made nano-scaled architectures for optical, electrical and magnetic applications.1–4 Moreover, the control and understanding of (switchable) surface properties are crucial for the development of advanced materials with well-defined wetting properties. Such so-called smart surfaces have already been used in applications as self-cleaning surfaces, tunable optical lenses, lab-on-chip systems, microfluidic devices and for different textile applications.5–10 Considerable effort has been made to understanding the influence of designed, rough surfaces on the wetting properties, initiated by the pioneering works of Wenzel, Cassie and Baxter some time ago.11,12 In several recent studies, raspberry-architectures consisting of a larger core particle surrounded by surface-attached smaller particles have especially been observed to provide high and adjustable surface roughness and high specific surface areas. This effect is also referred to as the “lotus effect”, and such materials are appealing for superhydrophobic coatings and films.13–18 Generally, raspberry-like particles have been obtained via Pickering emulsion polymerization,19,20 miniemulsion polymerization,21–23 heterocoagulation24,25 or direct hydrolysis of an inorganic precursor at the particle surface.26,27 An interesting and convenient approach for formation of raspberry-type structures was recently reported by Zhao and Middelberg by taking advantage of microfluidic synthetic protocols.28
In general, modification of monodisperse particles proves feasible, if bifunctional monomers (e.g. bearing both a vinylic and an epoxy functionality) are used, maintaining at least one reactive moiety subsequent to polymerization. For this purpose, especially the bifunctional glycidyl methacrylate (GMA) has been used as a monomer in order to prepare core/shell architectures or gels with reactive epoxy groups for further functionalization. The advantage of using epoxy groups is their convenient conversion into diols, amines or aldehydes. Hence, GMA has been polymerized and used for subsequent protein immobilization,29,30 drug delivery from PGMA gels,31 for liquid chromatography,32,33 for self-healing materials,34,35 for the formation of macroporous hydrogels,36 as a polylactide toughening agent,37 as membrane adsorber38 or for photonic crystals.39 GMA has also been used in order to produce functional (hybrid) core/shell and raspberry-like architectures for different applications.40–47
Recently, the novel bifunctional monomer vinylphenylglycidyl ether (VPGE) bearing both styrenic and epoxy functionalities has been presented in a patent.48 Compared to PGMA, PVPGE features superior thermal stability and a higher chemical and hydrolytic stability. In this work we present a strategy for the one-pot preparation of VPGE-based functional core/shell particles using a seeded emulsion polymerization approach, which is advantageous compared to established procedures. Moreover, another focus of this work is the quantification of the reactive epoxy sites at the latex particles. The epoxy moieties in the particle shell have also been employed for subsequent crosslinking reactions. Functional, hierarchically structured raspberry-like particles have been developed and deposited on surfaces in order to adjust wettability, aiming at highly hydrophobic surfaces.
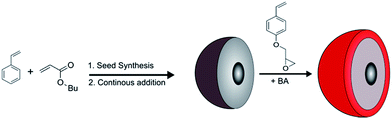
In order to prepare core/shell particles featuring a reactive functional shell based on VPGE, semi-continuous and stepwise emulsion polymerization protocols were applied, as depicted in Fig. 1 and described in the experimental section (ESI†).
This procedure allows precise control over the polymer composition and the shell constitution.49–51 For this purpose, poly(butyl acrylate) (PBA) seeds were synthesized in a batch process in the first step followed by the continuous addition of styrene (S) and butyl acrylate (BA) in the second step. VPGE bearing epoxy moieties was introduced in the last step of the particle synthesis by adding a mixture of VPGE and BA, as is also described in the experimental section. In order to maintain the epoxy functionalities without significant ring-opening reaction, a mixture of sodium dihydrogen phosphate and sodium hydrogen carbonate was used as a buffer system during the seeded emulsion polymerization. The success of the core/shell particle synthesis was verified by transmission electron microscopy (TEM) measurements for the final particle dispersion (Fig. 2, left). A corresponding TEM image of the soft core particles is given in the ESI Fig. S1.†
In addition to TEM measurements, dynamic light scattering (DLS) measurements for all particle synthesis steps were carried out (Fig. 2, right), furnishing proof for the successful generation of PVPGE-containing core/shell particles, as confirmed by the increasing average particle diameter. Moreover, corresponding particle size distributions after each reaction step revealed that monodisperse particles with a final average diameter of 348 nm were obtained. Values for the obtained average particle diameters for all syntheses steps are compiled in Table S2 of the ESI.†
It is a key issue, whether the epoxide moieties of the VPGE-units are still present and reactive subsequent to emulsion polymerization. For this purpose, two strategies were followed: (i) UV/vis spectroscopy measurements in an isorefractive solvent for the particle dispersion after attachment of a dye and (ii) differential scanning calorimetry (DSC) measurements of VPGE-core/shell particles in the presence of a crosslinker, as described in the ensuing section. In general, determination of the amount of functional groups at organic micro- and nanoparticles is a challenging task and typical analytical methods, such as elemental analysis, attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR), X-ray photoelectron spectroscopy (XPS), solid-state NMR, or calorimetric, colorimetric and fluorometric methods were used.52,53 Recently, we were able to show that transferring originally invisible ATRP initiators attached to organic particles into a RAFT chain transfer agent entity with a typical absorption band is a valuable technique in order to determine the amount of accessible ATRP-initiator by using UV/vis spectroscopy.52,54 For this purpose it was necessary to use an isorefractive dispersion medium for the investigated nanoparticles. Notably, accessibility in this particular case strongly depends on the size of the compounds to be attached and the molecular and local environment.
In the current study we choose 4-(4-nitrobenzyl)pyridine (NBP) – also referred to as the Preussmann Reagent – for efficient ring-opening reaction of epoxy functionalities (Fig. 3).55−57
 | ||
| Fig. 3 Reaction of surface-immobilized epoxy moieties with 4-(4-nitrobenzyl)pyridine (NBP, Preussmann reagent) for organic core/shell particle coloration. | ||
Ring-opening reaction of NBP with surface-attached epoxy moieties at the organic particle surface or shell, respectively, results in an intensive purple coloration. Recently, Schönherr and co-workers successfully applied the Preussmann test for determining the amount of epoxy functionalities at various inorganic substrates.55 We now expand these findings to the determination of epoxy functionalities in the exterior of a polymeric particle shell by using anisole as an isorefractive solvent for the particle dispersion, relying on UV/vis spectroscopy. Exemplarily, images of the Preussmann reagent in a solution of ethanol, particle dispersion after treatment with the Preussmann reagent and the corresponding diluted particle dispersion in an isorefractive solvent are shown in Fig. S3 of the ESI.† By further dispersing the pristine particle dispersion in anisole, the particles became nearly invisible, since the refractive index of the organic particles and the solvent (refractive indexanisole = 1.516) are very close. Consequently, the recorded UV/vis absorption spectra of the heterogeneous system indicated a suppression of particle scattering, enabling the quantification of epoxy functionalities (Fig. S4†). A detailed calculation of accessible epoxy functionalities after reaction with NBP is described in the ESI.† The molar extinction coefficient ε for NBP in anisole at 544 nm was determined to 1537.36 cm2 mmol−1 from the corresponding calibration plot (Fig. S5†). After reaction with NBP, the amount of accessible epoxy functionalities, cchromo, could be determined by UV/vis measurements. By assuming an average particle diameter of 350 nm, a value of 5.3 epoxide moiety per nm2 could be calculated, which is in excellent agreement with expectation.
To further confirm the maintained reactivity of surface-attached epoxides at the particles subsequent to emulsion polymerization, exothermic ring-opening reactions of PVPGE-containing core/shell particles with 3-aminomethyl-3,5,5-trimethylcyclohexylamine as a commercially available cross-linking reagent – also referred to as isophorone diamine (IPDA) – were studied using DSC measurements. Samples with different amounts of epoxy functionalities in the particle shell were treated with IPDA, as described in the experimental section (ESI† and Fig. S6†). In the first DSC run, an exothermic peak at approximately 70 °C due to the crosslinking reaction was obvious (Fig. S7,† top) demonstrating that immobilized epoxy functionalities were capable of undergoing ring-opening reaction with IPDA. Furthermore, in the second subsequent DSC experiment (Fig. S7,† bottom) of the same sample, a significant shift of the original glass transition temperature of 58 °C for the pristine organic particles (dotted line in Fig. S5†) to 95 °C was observed. These results additionally evidence fast and efficient ring-opening reaction of PVPGE-containing particles with IPDA. The possibility of subsequent crosslinking due to the presence of reactive epoxy-groups in the particle shell is an important tool for additional particle modification.
The PVPGE-containing core/shell particles were functionalized with smaller silica particles in order to generate raspberry-like particle architectures (Fig. 4). To this end, PVPGE-containing core particles with a high concentration of accessible surface epoxide moieties were slowly added to amino-functionalized silica particles. For this purpose, dropwise addition of a diluted core particle dispersion (0.13 wt% in ethanol) to the silica nanoparticle dispersion (2 wt% in ethanol) was used, resulting in a ring-opening reaction of the epoxide moieties by the surface-anchored amine groups. Average diameters of the silica nanoparticles employed were determined to 50 ± 7 nm and 120 ± 14 nm, respectively, by using DLS (Fig. S8†) and to 48 ± 5 nm and 104 ± 5 nm, respectively, by using TEM measurements (Fig. S9†).
 | ||
| Fig. 4 Reaction of PVPGE-containing core/shell particles bearing reactive epoxy functionalities and amino-functionalized silica particles for the preparation of raspberry-like hybrid architectures. | ||
Subsequent to this reaction, the excess of the smaller silica nanoparticles was removed by centrifugation, yielding rather pure hybrid raspberry-like particles consisting of an organic core and a large number of smaller silica particles. The colloidal organic–inorganic “raspberries” created have been investigated by TEM and SEM measurements (Fig. 5).
For surface modification and contact angle (CA) measurements, the raspberry-like particles (348 nm PVPGE particles with 120 nm silica particles) were deposited on glass wafers by coating with a doctor blade, (details see experimental section). After exposure to oxygen plasma in order to generate homogeneously distributed hydroxyl functionalities, the film was treated with dichlorodimethylsilane for hydrophobization. Both the entire particle film as well as the particle structure remained stable, as proven by SEM measurements (Fig. S10†). In order to study the wetting properties of the silane-treated hybrid particle films, static CA measurements against water were carried out (Fig. 6).
Results of the static CA for the hydrophobized raspberry-type structures were directly compared to the hydrophobized glass wafers after functionalization with dichlorodimethylsilane and values were found to increase from 98.6° ±3.1° for the reference sample up to 148.6° ± 1.7° for the raspberry-particle coated glass wafers. Noteworthy, CA measurements were carried out at 10 different positions of the glass wafer, revealing nearly identical CA values. These results evidence the suitability for herein prepared and investigated raspberry-type colloidal particles as highly water-repellent surface coatings.
In conclusion, a facile and straightforward synthetic procedure for the preparation of poly(vinylphenylglycidyl ether) (PVPGE)-containing core/shell latex particles has been developed that possess a high amount of reactive epoxy moieties at the surface. The presence of reactive epoxy functionalities was proven by (i) reaction with the Preussmann reagent (NBP) and determination of colored functional groups using UV/vis spectroscopy in anisole as an isorefractive solvent and (ii) by investigating the reaction of isophorone diamine (IPDA) with PVPGE-containing core/shell particles via differential scanning calorimetry (DSC). The addition of silica nanoparticles with amine functionalities was employed for the preparation of raspberry-like organic–inorganic hybrid particles with organic core (348 nm) and smaller silica particles as shell. After surface deposition, plasma treatment and silanization, the raspberry-like particles deposited on glass substrates led to excellent static contact angles against water on the order of 150°, remarkably without the use of fluorinated compounds as hydrophobic coating agents. In summary, these findings represent a facile approach for the preparation of hierarchical particle architectures suitable for highly hydrophobic surfaces. Moreover, the synthesis of the novel raspberry-like architectures and coating strategies can be exploited as excellent and facile platform for the preparation of ultra-hydrophobic surfaces with adjustable surface roughness which is currently under investigation. Furthermore, this work evidences the potential of the novel bifunctional styrene monomer VPGE that can readily be combined with styrene and a broad range of other vinyl monomers. The highly epoxide-functional organic latex particles are also promising for photonic films, as toughening agents as well as materials for chemical sensing applications.
Acknowledgements
The authors thank Daniel Scheid for support for the particle film preparation, Jurema Schmidt (Juju) for help with particle synthesis and the group of Prof. Markus Biesalski from the Macromolecular Chemistry and Paper Department (TU Darmstadt) for help with CA measurements. We acknowledge the Landesoffensive zur Entwicklung Wissenschaftlich-Ökonomischer Exzellenz (LOEWE) of the State of Hesse through the research initiative Soft Control for ongoing financial support.Notes and references
- M. E. Davis, Nature, 2002, 417, 813–821 CrossRef CAS PubMed.
- C. Burda, X. Chen, R. Narayanan and M. A. El-Sayed, Chem. Rev., 2005, 105, 1025–1102 CrossRef CAS PubMed.
- Y. Zhao and L. Jiang, Adv. Mater., 2009, 21, 3621–3638 CrossRef CAS PubMed.
- C. G. Schäfer, S. Vowinkel, G. P. Hellmann, T. Herdt, C. Contiu, J. J. Schneider and M. Gallei, J. Mater. Chem. C, 2014, 2, 7960–7975 RSC.
- W. Sun, S. Zhou, B. You and L. Wu, J. Mater. Chem. A, 2013, 1, 3146–3154 CAS.
- F. Shi, Y. Song, J. Niu, X. Xia, Z. Wang and X. Zhang, Chem. Mater., 2006, 18, 1365–1368 CrossRef CAS.
- C. L. Feng, Y. J. Zhang, J. Jin, Y. L. Song, L. Y. Xie, G. R. Qu, L. Jiang and D. B. Zhu, Langmuir, 2001, 17, 4593–4597 CrossRef CAS.
- Y. Pei, J. Travas-Sejdic and D. E. Williams, Langmuir, 2012, 28, 8072–8083 CrossRef CAS PubMed.
- J. Hu, H. Meng, G. Li and S. I. Ibekwe, Smart Mater. Struct., 2012, 21, 053001 CrossRef.
- J. Drelich, E. Chibowski, D. D. Meng and K. Terpilowski, Soft Matter, 2011, 7, 9804–9828 RSC.
- R. N. Wenzel, Ind. Eng. Chem. Res., 1936, 28, 988–994 CrossRef CAS.
- A. B. D. Cassie and S. Baxter, Trans. Faraday Soc., 1944, 40, 0546–0550 RSC.
- W. Ming, D. Wu, R. v. Benthem and G. d. With, Nano Lett., 2005, 5, 2298–2301 CrossRef CAS PubMed.
- W. Jiang, C. M. Grozea, Z. Shi and G. Liu, ACS Appl. Mater. Interfaces, 2014, 6, 2629–2638 CAS.
- B. Peng, L. Tan, D. Chen, X. Meng and F. Tang, ACS Appl. Mater. Interfaces, 2012, 4, 96–101 CAS.
- T. Ribeiro, C. Baleizão and J. Farinha, Materials, 2014, 7, 3881–3900 CrossRef PubMed.
- L. A. Fielding, J. Tonnar and S. P. Armes, Langmuir, 2011, 27, 11129–11144 CrossRef CAS PubMed.
- H.-J. Tsai and Y.-L. Lee, Langmuir, 2007, 23, 12687–12692 CrossRef CAS PubMed.
- A. Schrade, Z. Cao, K. Landfester and U. Ziener, Langmuir, 2011, 27, 6689–6700 CrossRef CAS PubMed.
- Z. Nie, J. I. Park, W. Li, S. A. F. Bon and E. Kumacheva, J. Am. Chem. Soc., 2008, 130, 16508–16509 CrossRef CAS PubMed.
- F. Tiarks, K. Landfester and M. Antonietti, Langmuir, 2001, 17, 5775–5780 CrossRef CAS.
- Y. Zhang, H. Chen, X. Shu, Q. Zou and M. Chen, Colloids Surf., A, 2009, 350, 26–32 CrossRef CAS PubMed.
- S.-W. Zhang, S.-X. Zhou, Y.-M. Weng and L.-M. Wu, Langmuir, 2005, 21, 2124–2128 CrossRef CAS PubMed.
- B. Zhao and M. M. Collinson, Chem. Mater., 2010, 22, 4312–4319 CrossRef CAS.
- H. Minami, Y. Mizuta and T. Suzuki, Langmuir, 2013, 29, 554–560 CrossRef CAS PubMed.
- M. Agrawal, A. Pich, S. Gupta, N. E. Zafeiropoulos, P. Formanek, D. Jehnichen and M. Stamm, Langmuir, 2010, 26, 526–532 CrossRef CAS PubMed.
- M. Agrawal, A. Pich, N. E. Zafeiropoulos, S. Gupta, J. Pionteck, F. Simon and M. Stamm, Chem. Mater., 2007, 19, 1845–1852 CrossRef CAS.
- C.-X. Zhao and A. P. J. Middelberg, RSC Adv., 2013, 3, 21227–21230 RSC.
- B. Chen, N. Pernodet, M. H. Rafailovich, A. Bakhtina and R. A. Gross, Langmuir, 2008, 24, 13457–13464 CrossRef CAS PubMed.
- H. Hlidkova, D. Horak, V. Proks, Z. Kucerova, M. Pekarek and J. Kucka, Macromol. Biosci., 2013, 13, 503–511 CrossRef CAS PubMed.
- P. Li, R. Xu, W. Wang, X. Li, Z. Xu, K. W. Yeung and P. K. Chu, Colloids Surf., B, 2013, 101, 251–255 CrossRef CAS PubMed.
- F. Limé and K. Irgum, Macromolecules, 2009, 42, 4436–4442 CrossRef.
- Y. Lv, F. M. Alejandro, J. M. Frechet and F. Svec, J. Chromatogr. A, 2012, 1261, 121–128 CrossRef CAS PubMed.
- M. Ghaemy and S. Bekhradnia, J. Appl. Polym. Sci., 2010, 117, 467–472 CAS.
- J. Lu, A. J. Easteal and N. Edmond, J. Appl. Polym. Sci., 2012, 126, 172–181 CrossRef PubMed.
- S. Zhou, A. Bismarck and J. H. G. Steinke, J. Mater. Chem., 2012, 22, 18824–18829 RSC.
- S. Sun, M. Zhang, H. Zhang and X. Zhang, J. Appl. Polym. Sci., 2011, 122, 2992–2999 CrossRef CAS PubMed.
- F. Radovanović, A. Nastasović, T. Tomković, D. Vasiljević-Radović, A. Nešić, S. Veličković and A. Onjia, React. Funct. Polym., 2014, 77, 1–10 CrossRef PubMed.
- Z. Luo, C. Zou, S. Syed, L. A. Syarbaini and G. Chen, Colloid Polym. Sci., 2011, 290, 141–150 Search PubMed.
- W. Zhao, R. J. Yang, T. T. Qian, X. Hua, W. B. Zhang and W. Katiyo, Int. J. Mol. Sci., 2013, 14, 12073–12089 CrossRef PubMed.
- P. Tao, Y. Li, A. Rungta, A. Viswanath, J. Gao, B. C. Benicewicz, R. W. Siegel and L. S. Schadler, J. Mater. Chem., 2011, 21, 18623–18269 RSC.
- P. Tao, A. Viswanath, Y. Li, R. W. Siegel, B. C. Benicewicz and L. S. Schadler, Polymer, 2013, 54, 1639–1646 CrossRef CAS PubMed.
- W. L. Zhang, S. H. Piao and H. J. Choi, J. Colloid Interface Sci., 2013, 402, 100–106 CrossRef CAS PubMed.
- J. Oh, J.-H. Lee, J. C. Koo, H. R. Choi, Y. Lee, T. Kim, N. D. Luong and J.-D. Nam, J. Mater. Chem., 2010, 20, 9200–9204 RSC.
- X. Fan, X. Jia, H. Zhang, B. Zhang, C. Li and Q. Zhang, Langmuir, 2013, 29, 11730–11741 CrossRef CAS PubMed.
- N. Puretskiy and L. Ionov, Langmuir, 2011, 27, 3006–3011 CrossRef CAS PubMed.
- Y. Liu, M. Li and G. Chen, J. Mater. Chem. A, 2013, 1, 930–937 CAS.
- K. Kunitsky, M. C. Shah, S. W. Shuey and M. E. Wagman, US 2008/0167433 A1 2008.
- C. G. Schäfer, D. A. Smolin, G. P. Hellmann and M. Gallei, Langmuir, 2013, 29, 11275–11283 CrossRef PubMed.
- D. Scheid, C. Lederle, S. Vowinkel, C. G. Schäfer, B. Stühn and M. Gallei, J. Mater. Chem. C, 2014, 2, 2583–2590 RSC.
- C. G. Schäfer, M. Gallei, J. T. Zahn, J. Engelhardt, G. P. Hellmann and M. Rehahn, Chem. Mater., 2013, 25, 2309–2318 CrossRef.
- M. Mazurowski, M. Gallei and M. Rehahn, ACS Macro Lett., 2012, 1, 1362–1366 CrossRef CAS.
- A. Hennig, H. Borcherding, C. Jaeger, S. Hatami, C. Würth, A. Hoffmann, K. Hoffmann, T. Thiele, U. Schedler and U. Resch-Genger, J. Am. Chem. Soc., 2012, 134, 8268 CrossRef CAS PubMed.
- M. Mazurowski, M. Gallei, J. Li, H. Didzoleit, B. Stühn and M. Rehahn, Macromolecules, 2012, 45, 8970–8981 CrossRef CAS.
- P. Schönherr, A. Seifert, R. Lungwitz, F. Simon, N. Moszner, P. Burtscher and S. Spange, Prog. Org. Coat., 2012, 75, 335–343 CrossRef PubMed.
- R. Preussmann, H. Schneider and F. Epple, Arzneim. Forsch., 1969, 19, 1059–1073 CAS.
- E. Zyner, J. Graczyk and J. Ochocki, Pharmazie, 1999, 54, 945–946 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Additional data on materials, instrumentation, general experimental procedures, DSC experiments, silica particle syntheses, detailed calculation of epoxy functionalities, TEM images, photographs of the particle dispersion after NBP treatment, UV/vis spectra, calibration plots for UV/vis measurements, additional DLS measurements, SEM images of raspberry-particle films. See DOI: 10.1039/c4ra08382b |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2014 |