Self-assembly of a biodegradable branched PE-PCL-b-PEC amphiphilic polymer: synthesis, characterization and targeted delivery of doxorubicin to cancer cells†
S. Panjaa,
S. Nayakb,
S. K. Ghoshb,
M. Selvakumara and
S. Chattopadhyay*a
aRubber Technology Centre, Indian Institute of Technology, Kharagpur-721302, India. E-mail: santanuchat71@yahoo.com
bDepartment of Biotechnology, Indian Institute of Technology, Kharagpur-721302, India
First published on 30th September 2014
Abstract
A novel biodegradable branched block copolymer was synthesized by the ring-opening polymerization of ethylene carbonate using pre-synthesized four-armed pentaerythretol poly(ε-caprolactone) (PE-PCL) as a macro initiator. Folic acid was conjugated with the end-group of the block copolymer and self-assembled in water to form polymer micelles (PMs). The very low critical micelle concentration of the block copolymer suggests its potential application in advanced drug delivery systems. The PMs are spherical in shape and have an average size of 80 nm, which is suitable for the delivery of drugs. The hydrophobicity of pentaerythretol poly(ε-caprolactone) and its branched structure can accommodate high amounts of doxorubicin. Compared with a blank sample, PMs containing encapsulated doxorubicin show a much higher cytotoxicity towards HeLa cells. A high rate of release of doxorubicin in vitro at pH 5.0 shows that the system is responsive to pH. Confocal laser scanning microscopy showed that the doxorubicin-loaded PMs were internalized into the HeLa cells.
Introduction
Nanocarriers are used extensively to deliver drug molecules to specific sites in the body. This approach is often used to decrease the side-effects of highly toxic drugs with a low solubility in water.1 Biocompatible/biodegradable polymer micelles (PMs) are one of the most promising nanocarriers for targeted drug delivery.2 The PMs used for the targeted delivery of drugs must have the following characteristics: (a) they must be stable in phosphate-buffered saline (PBS) (pH 7.4); (b) they must be able to encapsulate, carry and deliver the drug; (c) they should be biocompatible and/or biodegradable inside the biological system; (d) their size should be in the range 10–100 nm for effective performance;3,4 and (e) they must be site-specific.5Biocompatible/biodegradable amphiphilic polymers are widely used for the preparation of such PMs. Amphiphilic polymers are mainly formed by tailoring blocks of hydrophilic and hydrophobic polymers,6,7 i.e. by incorporating hydrophilic functional groups onto the backbone of a hydrophobic polymer, or by incorporating hydrophobic groups onto the backbone of a hydrophilic polymer. The hydrophobic parts of these amphiphilic polymers serve as efficient shields with which to hold the toxic drug molecules, which usually have a low solubility in water. In contrast, the hydrophilic part of these amphiphilic polymers is responsible for the colloidal stability of the polymer nanoparticles in water. Poly(lactic acid-co-glycolic acid),8,9 polycaprolactone (PCL),6,10–14 polyphosphazene,15 poly(propylene fumarate),16 chitosan,17,18 and their modified variants are widely used to design the hydrophobic part of amphiphilic polymers. Polyethylene glycol (PEG)19–21 is one of the polymers most widely used as the hydrophilic part of amphiphilic polymers.
The PMs formed by linear amphiphilic poly(ethylene glycol)-b-poly(ε-caprolactone-co-γ-hydroxyl-ε-caprolactone) have been used as nanocarriers to deliver hydrophobic anticancer drugs, including doxorubicin (DOX).22 Free DOX has a higher cytotoxicity than DOX-loaded PMs, indicating the effective encapsulation of the drug into the PMs. Liu et al.23 reported the synthesis of docetaxel-loaded PMs using poly(ethylene glycol)-b-poly(caprolactone) and subsequently investigated its antitumor action. They also reported that the particles formed in this way had a high degree of penetration into the tumor cell. The linear amphiphilic triblock copolymer formed by hydrophilic PEG and hydrophobic oxime-tethered PCL has also been used as an efficient nanocarrier for delivering anticancer drugs.24 A special type of amphiphilic block copolymer was synthesized via the supramolecular interaction of adenine and uracil groups attached at the end of the hydrophobic PCL and hydrophilic PEG parts of the polymer, respectively.25 This non-covalently connected amphiphilic polymer acts as a stimuli-responsive (pH-responsive) carrier for anticancer drugs. Compared with linear polymers, branched and hyperbranched analogues appear to be more useful as nanocarriers for drug delivery, as confirmed by a recent study by Yong et al.4 on a Y-shaped polymer – a three-armed thermo-responsive amphiphilic block copolymer prepared from hydrophobic poly(undecylenic acid) and hydrophilic poly(N-isopropylacrylamide). This polymer was subsequently successfully used for thermo-responsive drug delivery. In addition, two Y-shaped thermo-responsive amphiphilic copolymers based on poly(ε-benzyloxycarbonyl-L-lysine) and poly(L-lysine) were synthesized by Li-Ying et al.26 and used for drug delivery. The synthesis of the Y-shaped poly(L-lactide)2-b-poly(g-benzyl-L-glutamic acid) copolymer and the formation of its self-assembled form were reported by Sun et al.27 A polyamidoamine dendrimer was synthesized by Singh et al.28 and used for the delivery of anticancer drugs. A polyester-based amphiphilic hyperbranched polymer was reported by Chen et al.29,30 It was synthesized by extending the chains of the commercially available hyperbranched aliphatic polyester Boltorn H40 with PCL and then coupled with PEG. A self-assembled fluorine-based hyperbranched polymer was synthesized by Du et al.19 as an agent for use in functional MRI.31,32
Unlike linear polymers, branched or hyperbranched polymers have many advantages, such as a high efficiency of encapsulation, the ability to form templates33 and high functionality, leading to a wide range of interactions with biological systems. In the work reported here, we attempted to synthesize PMs from branched amphiphilic block copolymers to combine all these advantages of branched polymers. Most of the earlier publications have reported amphiphilic polymers prepared from PCL as the hydrophobic segment and PEG as the hydrophilic segment. Although PEG is widely used as a hydrophilic polymer for drug delivery, it still has certain limitations, such as (a) a potential hypersensitivity reaction (it may produce anaphylactic shock) by PEG itself or by side products formed during polymerization,34 (b) abnormalities in its pharmacokinetic behavior35 and (c) its non-biodegradability.36
We aimed to overcome these limitations of existing nanocarrier systems by replacing PEG with polyethylene carbonate (PEC). PEC is well known for its biodegradability37,38 and is also biocompatible in nature. PEG is more hydrophilic than PEC; however, PEC is more hydrophilic (water solubility 88.1 g L−1 at 20 °C; Sigma-Aldrich MSDS) than PE-PCL. This confirms the amphiphilicity of the desired block copolymer. The PE-initiated four-armed PE-PCL was synthesized by the simple ring-opening polymerization of ε-caprolactone (CL). The amphiphilic block copolymers of PEC (PE-PCL-b-PEC) were synthesized by the ring-opening polymerization of different equivalent amounts of ethylene carbonate (EC) using four-armed PE-PCL as a macro initiator. To target cancer cells, the block copolymer was tethered with folic acid (FA). The structures of the synthesized homo-, block and FA-tethered polymers were established by 1H-NMR spectrometry. The water contact angle of the synthesized block copolymer was measured by a contact angle goniometer. The FA-attached amphiphilic polymer (PE-PCL-b-PEC-FA) was then precipitated in water to form the PMs. The hydrodynamic size and bulk morphology of the PMs were studied by dynamic light-scattering spectroscopy (DLS) and HRTEM. The critical micelle concentration (CMC) of the PMs was determined by fluorescence spectroscopy using pyrene as a fluorescent probe. The drug-loading efficiency (DLE), drug-loading content (DLC) and release profile of an anticancer drug (DOX) were studied by UV spectroscopy. We also studied the effectiveness of DOX-loaded PMs towards specific uptake by cancer cells (HeLa cells) and the release and cytotoxicity of DOX.
Experimental section
Characterizations
The structure of the synthesized homo-block copolymer and the FA-attached block copolymer were confirmed by 1H-NMR spectrometry using a Bruker Avance instrument operating at 200 MHz. The UV-visible absorption spectra of the FA-attached block copolymer were recorded by dissolving the sample in DMSO and scanning from 700 to 200 nm using a Perkin-Elmer Lambda 35 instrument at a slit width of 1 nm. Differential scanning calorimetry was used to study the thermal properties of the block copolymer using a DSC Q100 V8.1 Build 251 thermogravimeter. The samples were heated from +25 °C to +100 °C at a heating rate of 20 °C min−1, cooled down to −100 °C at a rate of 20 °C min−1 and then heated again to +100 °C at the same rate. The second heating cycle was used to determine the Tg and Tm of the block copolymer.The molecular weight and its distribution were examined by size-exclusion chromatography. The molecular weights of the polymers were determined at ambient temperature using a Viscotek gel permeation chromatograph equipped with a VE 1122 solvent-delivery system, a VE 3580 RI detector and two Visco GEL mixed-bed columns (17392-GMHHRM), which were preceded by a guard column. HPLC-grade THF was used as the eluent at a flow-rate of 1.0 mL min−1 and calibration was carried out using low polydispersity poly(methyl methacrylate) standards.
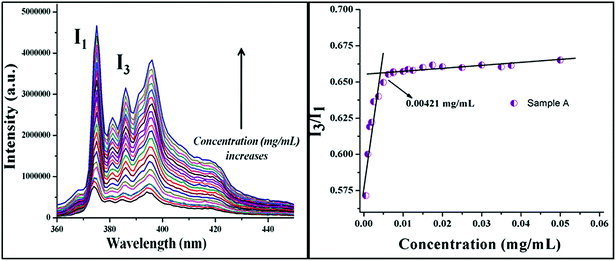
The CMCs of the PMs were determined by taking a constant amount of pyrene as a fluoroprobe and varying the concentration of the polymer from 0.001 to 0.1 mg mL−1. The fluorescence spectra were recorded using a Jobin Yvon-Spex Fluorolog-3 spectrofluorimeter equipped with a thermostatic cell holder with a 1 cm path length quartz cuvette. An excitation wavelength of 325 nm was used in all instances for the selective excitation of pyrene and the emission spectra were recorded from 360 to 600 nm. The CMCs were evaluated by plotting the ratio of I3 and I1 against concentration.
The bulk morphology of the PMs was studied by HRTEM using a JEOL 2000 instrument operated at an accelerating voltage of 200 kV. The hydrodynamic size of the PMs was determined by DLS using a Malvern Nano ZS instrument. The experiment was conducted using a 4 mW He–Ne laser (λ = 632.8 nm) at 298 K. The detector angle was fixed at 90° in a Zetasizer Nano ZS instrument. The phase image of the PMs was evaluated by an AFM Nd:YAG laser scanner (Agilent Technologies, Multiview-1000; 70 μm AFM/NSOM) in the tapping mode using silicon cantilevers. The water contact angle of the synthesized polymers was measured by a contact angle goniometer (Rame-Hart instrument Co., Model 190F2). The synthesized polymer film was cast onto a glass slide for the contact angle measurements.
Materials
Pentaerythritol (PE), ε-caprolactone (CL), tin(II) ethylhexanoate [Sn(Oct)2], EC, FA, N,N′-dicyclohexylcarbodiimide (DCC) and doxorubicin hydrochloride (DOX) were purchased from Sigma-Aldrich, USA. N-Hydroxysuccinimide was purchased from Spectrochem (Mumbai, India). The solvents DMF, toluene and hexane, all of analytical-reagent grade, were purchased from Merck Chemical (Mumbai, India).Synthesis of branched PE-PCL
Branched PE-PCL was synthesized by ring-opening polymerization, as reported previously.39 PE was taken into a 25 mL two-necked round-bottomed flask (RBF) under an N2 atmosphere. The required amount of monomer (CL) was added to the RBF, which was then placed into a preheated oil-bath at 120 °C. After homogenization of the reaction mixture, the required amount of catalyst [Sn(Oct)2 at a constant 1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w ratio of monomer and catalyst] was added to the reaction mixture and the reaction was allowed to continue at the same temperature until 97–98% conversion (confirmed by a gravimetric method); after this level of conversion, the reaction mixture appeared highly viscous. The reaction mixture was then cooled down to room temperature. The resulting solid mass was dissolved in a minimum volume of chloroform and precipitated into hexane. The precipitated product was dried under vacuum at 60 °C under reduced pressure.
1 w/w ratio of monomer and catalyst] was added to the reaction mixture and the reaction was allowed to continue at the same temperature until 97–98% conversion (confirmed by a gravimetric method); after this level of conversion, the reaction mixture appeared highly viscous. The reaction mixture was then cooled down to room temperature. The resulting solid mass was dissolved in a minimum volume of chloroform and precipitated into hexane. The precipitated product was dried under vacuum at 60 °C under reduced pressure.
Synthesis of branched PE-PCL-b-PEC
PE-PCL-b-PEC was synthesized by the ring-opening polymerization of EC40 using branched PE-PCL as a macro initiator. The calculated amount of PE-PCL and EC were taken into a 25 mL two-necked RBF fitted with a condenser under a dry N2 atmosphere. The required amount of dry toluene (at a constant 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 w/v ratio of EC and toluene) was then added. After homogenization of the reaction mixture, the RBF was placed into a preheated oil-bath at 110 °C. The calculated amount of catalyst [Sn(Oct)2 at a constant 1000
10 w/v ratio of EC and toluene) was then added. After homogenization of the reaction mixture, the RBF was placed into a preheated oil-bath at 110 °C. The calculated amount of catalyst [Sn(Oct)2 at a constant 1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w ratio of EC and catalyst] was injected into the RBF just after starting reflux of toluene. The reaction was continued for up to 20 h at the same temperature. Finally, the reaction mixture was precipitated into diethyl ether and dried in a vacuum oven under reduced pressure.
1 w/w ratio of EC and catalyst] was injected into the RBF just after starting reflux of toluene. The reaction was continued for up to 20 h at the same temperature. Finally, the reaction mixture was precipitated into diethyl ether and dried in a vacuum oven under reduced pressure.
Synthesis of FA-tethered PE-PCL-b-PEC copolymer
The FA-tethered PE-PCL-b-PEC copolymer was synthesized using a slight modification of a previously reported procedure (Scheme 1).41–43 FA (4 equiv.) with DCC (4.5 equiv.) and N-hydroxysuccinimide (4.5 equiv.) was dissolved in DMF in a two-necked RBF under an N2 atmosphere. The reaction mixture was cooled down from room temperature using an ice-bath. The block copolymer (1 equiv.) dissolved in DMF was then slowly added into the RBF containing FA. After 1 h, the ice-bath was removed from the reaction system and stirring was continued for up to 24 h at room temperature. The FA-tethered polymer was then filtered through a filter-paper to remove any dicyclohexylcarbodiimide (DCU). The filtrate was dialyzed against DMF through a dialysis membrane (cut-off mol. wt 3.5 kD; cellulose acetate membrane) to remove any unreacted FA. The FA-tethered polymer was precipitated into an excess amount of hexane and dried under vacuum. The final product was characterized by 1H-NMR spectrometry.Preparation of FA-conjugated, DOX-entrapped PMs
The FA-conjugated block copolymer (10 mg) was dissolved in 100 μL of DOX solution (2.5 mg DOX/100 μL DMSO). The mixture of copolymer and DOX was stirred overnight in the dark. The resulting mixture was then purged slowly into 10 mL of double-distilled water with vigorous agitation. The hydrophobic nature of DOX means that it is preferentially entrapped in the hydrophobic core (PCL core) of the block copolymer (Scheme 2). The suspension of PMs with entrapped DOX was then transferred into a dialysis bag (cut-off mol. wt 3.5 kDa, cellulose acetate) and dialyzed against water to remove DMSO and unbound DOX. The dialysis was continued for up to 24 h at room temperature in the dark. The amount of DOX loaded into the polymer was calculated by taking the absorbance of a known amount of DOX-loaded polymer at 482 nm and from a calibration graph for the absorbance of free DOX. The calibration graph was constructed by adding different concentrations of DOX into DMSO. The DOX-loading content (the DLC) of the PM and the DOX-loading efficiency (the DLE) were calculated as follows:
 | (1) |
 | (2) |
Cytotoxicity assay
The in vitro cytotoxicity of free DOX and the DOX-loaded PM were investigated using both a normal cell line (L929) and a cancer cell line (HeLa). Both the cell lines were seeded into a 96-well plate at a concentration of 1 × 105 cells per well in 200 μL of Dulbecco's Modified Eagle's Medium (DMEM) with 10% fetal bovine serum and were allowed to grow for 24 h at 37 °C in 5% CO2. The medium was then replaced by a culture medium containing serial dilutions (in order of concentration) of free DOX and DOX-loaded PMs and was cultured for 24 h. After treatment with DOX and the DOX-loaded PMs, the medium was discarded and 200 μL of 1 mg mL−1 MTT reagent in PBS was added to each well and incubated for another 4 h. A formazan crystal was formed by the reduction of MTT with the mitochondrial dehydrogenase enzyme of various cells. The unreacted MTT solution was then replaced by 200 μL of DMSO solution and mixed thoroughly to dissolve the formazan crystals. The optical density (OD) was measured spectrophotometrically at 570 nm on a bench-top microplate reader. The cell viability was calculated as follows: (ODsample/ODcontrol) × 100.Cellular uptake study
The cellular uptake of the DOX-loaded PMs was determined by culturing L929 and HeLa cell lines separately in a 24-well plate with 400 μL of DMEM with 10% fetal bovine serum at a cell concentration of 3 × 103 cells per well at 37 °C for 24 h under 5% CO2. The culture medium was then removed from each well and refilled with the same volume of medium containing 14.84 μg mL−1 of DOX-loaded PMs. The cell lines were cultured with DOX-loaded PMs for 1 h, 2 h and 4 h under the same conditions. After the allotted time, the cells were washed three times with 1× PBS solution and fixed by ice-cooled 2% paraformaldehyde. The nuclei of the cells were counter-stained with DAPI (blue). To investigate the cellular uptake of the DOX-loaded PMs, the fixed cells were subjected to confocal laser scanning microscopy. Images were captured by exciting the sample with a 480 nm He–Ne laser and detection was with an Olympus confocal microscope (FV1000, Olympus).Results and discussion
Synthesis and characterization of branched PE-PCL
The branched PE-PCL was synthesized by the simple ring-opening polymerization technique described in the experimental section. The chemical structure of the branched polymer was confirmed by 1H-NMR spectrometry (Fig. S1†). The resonance signal at 4.3 ppm is assigned to the methylene proton (–CH2O–, a) of the initiator (PE). The spectrum shows two more signals at 4 ppm and 2.3 ppm that are assigned to the methylene protons (–CH2O–, e) and (–COCH2–, b) attached to the main chain. A signal at 3.6 ppm is assigned to the terminal methylene proton (–CH2OH, f) of the polymer chains. Two signals at 1.3 ppm and 1.6 ppm are assigned to their corresponding protons (d and c) in Fig. S1.†Synthesis and characterization of branched PE-PCL-b-PEC
The block copolymer was synthesized by the ring-opening polymerization of EC described in the experimental section. The chemical structure and composition of the block copolymer were confirmed by 1H-NMR spectrometry, GPC and DSC. The 1H-NMR spectrum shows a number of resonance signals at different chemical shifts (Fig. 1). The signals at 4 ppm, 2.3 ppm, 1.6 ppm and 1.3 ppm are assigned to the –CH2O– (b), –OCOCH2– (d), –CH2– (e) and –CH2– (f) protons of the PE-PCL sector of the block copolymer, respectively. The signals at 4.5 ppm and 3.6 ppm are assigned to the –CH2– (a) and –CH2O– (c) protons of the EC part of the block copolymer.We synthesized block copolymers with different proportions of PE-PCL and EC. We used PE-PCL as a macro initiator with a fixed molecular weight (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 Da) and the amount of EC was varied (Table 1). The formation of the block copolymer with different molecular weights was confirmed by GPC (Fig. 2). The retention time of the block copolymers (samples A and B, Table 1) are shifted to the left (i.e. a lower retention time) compared with the homopolymer (PE-PCL). This shift in retention time recognizes the increase in molecular weight (compared with PE-PCL) and the formation of the block copolymers. The growth of the hydrophilic block (PEC) from the hydrophobic block (PE-PCL), i.e. the formation of the block copolymer, was also confirmed by the reduction in the water contact angle from 77° (for PE-PCL) to 55° (for sample D) (Fig. S2†). The formation of the block copolymer was confirmed by DSC (Fig. 3). The DSC thermogram of the homopolymer PE-PCL shows two melting peaks at 50 °C and 54 °C, respectively. These temperatures correspond to the main chain and end-group crystallization of the PE-PCL chains, respectively.39 The disappearance of the melting peak at 54 °C for the block copolymer is attributed to the growth of amorphous PEC chains from the end-group (–OH) of the homopolymer (PE-PCL).
300 Da) and the amount of EC was varied (Table 1). The formation of the block copolymer with different molecular weights was confirmed by GPC (Fig. 2). The retention time of the block copolymers (samples A and B, Table 1) are shifted to the left (i.e. a lower retention time) compared with the homopolymer (PE-PCL). This shift in retention time recognizes the increase in molecular weight (compared with PE-PCL) and the formation of the block copolymers. The growth of the hydrophilic block (PEC) from the hydrophobic block (PE-PCL), i.e. the formation of the block copolymer, was also confirmed by the reduction in the water contact angle from 77° (for PE-PCL) to 55° (for sample D) (Fig. S2†). The formation of the block copolymer was confirmed by DSC (Fig. 3). The DSC thermogram of the homopolymer PE-PCL shows two melting peaks at 50 °C and 54 °C, respectively. These temperatures correspond to the main chain and end-group crystallization of the PE-PCL chains, respectively.39 The disappearance of the melting peak at 54 °C for the block copolymer is attributed to the growth of amorphous PEC chains from the end-group (–OH) of the homopolymer (PE-PCL).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300; Đ= 1.2) as the macro initiator at 120 °C under an N2 atmosphere
300; Đ= 1.2) as the macro initiator at 120 °C under an N2 atmosphere
| Exp. no. | EC/PE-PCL (mol mol−1) | Mna (kDa) | Đa | PCL content (%) | CMCb (mg mL−1) × 10−3 | DLCc (%) | DLEd (%) | Sample code |
|---|---|---|---|---|---|---|---|---|
| a Evaluated from GPC analysis.b Determined by fluorescence spectrometry using a pyrene probe.c Determined from eqn (1).d Determined from eqn (2). | ||||||||
| 1 | 100 | 16.5 | 1.3 | ∼68 | 4.21 | 14.7 | 59.0 | A |
| 2 | 150 | 19.6 | 1.2 | ∼58 | 6.32 | 12.4 | 49.7 | B |
| 3 | 200 | 24.3 | 1.2 | ∼46 | 8.75 | 11.0 | 44.1 | C |
| 4 | 250 | 26.1 | 1.3 | ∼43 | 9.32 | 9.9 | 39.9 | D |
The emergence of a glass transition temperature at around 22 °C (the signature of PEC) along with a melting temperature of 50 °C (the signature of PE-PCL) in the DSC thermogram is attributed to the formation of the block copolymer (PE-PCL-b-PEC).
Synthesis and characterization of FA-conjugated PE-PCL-b-PEC copolymer
The FA-conjugated block copolymer was synthesized via the reaction of HO-PEC-b-PE-PCL with FA with the help of DCC coupling. The material between the α- and β-carboxylic acids of the FA-conjugated block copolymers has a superior biocompatibility with an increased circulation time in the bloodstream and allows the conjugate to act as an active targeting agent towards cancer cells.44 The conjugation of FA with the block copolymer was confirmed by 1H-NMR spectrometry (Fig. 4). The spectrum shows a number of resonating signals at 4.5 ppm, 4 ppm, 2.3 ppm, 1.6 ppm and 1.3 ppm, which are assigned to the –CH2– (a), –CH2O– (b), –OCOCH2– (c), –CH2– (d) and –CH2– (e) protons attached to the main chain carbons of the block copolymer. In addition, another set of resonating signals emerged between 6 and 9 ppm and was assigned to the protons of FA.42,45 We quantitatively determined the percentage of FA conjugation in the block copolymer using UV-visible spectrometry (Fig. S3†). Unlike FA, the pure block copolymer did not show any absorbance in the range 370–280 nm. However, the FA-conjugated block copolymer shows two absorption maxima at 280 and 365 nm, confirming the successful conjugation of FA with the block copolymer. An attachment of about 4% (by weight) of FA is confirmed from the absorption intensity of the FA-conjugated block copolymer at λ = 362 nm and from the calibration graph obtained with free FA in DMF.Preparation and characterization of FA-conjugated PMs
FA-conjugated PMs were prepared as described in the Experimental section; Fig. 5 shows the HRTEM and AFM images of the PMs. The average size of the PMs was 80 nm. The hydrodynamic size of the blank (98 ± 2 nm, Đ = 0.25) and DOX-loaded (111 ± 2 nm, Đ = 0.31) PMs (determined by DLS, Fig. S4†) were higher than those observed from the TEM photomicrographs; this may be because the TEM micrographs were captured under dry conditions that may lead the PMs to shrink to a smaller size compared with those obtained by DLS. PE-PCL-b-PEC-FA forms micelles with a water-holding dynamic structure and therefore the dilution stability of the PMs is determined by the CMC. The CMCs for these block copolymers were calculated by fluorescence spectrometry using pyrene as a fluorescence probe (Fig. 6). All the block copolymers show a CMC of the order of 10−3 mg mL−1 (Table 1). The CMC value shows a slightly increasing trend from sample A to sample D (Table 1). This is a result of the increase in the chain length of the hydrophilic component (PEC segment) from sample A to sample D.46 In general, the lower the value of the CMC, the better the dilution stability. Table 1 gives the DLC and DLE values determined by spectrometry. The DLC of the block copolymer shows a decreasing trend from sample A (14.7%) to sample D (9.9%). As we are dealing with a hydrophobic drug (DOX), this decrease in the DLC is attributed to the decrease in the percentage of the hydrophobic segment (PCL about 68% to 43%, i.e. an increase in the hydrophilic PEC segment) of the block copolymer from sample A to sample D.Cytotoxicity assay
To check the biocompatibility of the synthesized PMs, an MTT assay was performed against both cancerous (HeLa) and normal (L929) cell lines (Fig. 7). Samples B, D and the DOX-loaded sample D did not reach 50% inhibition of growth for the L929 cell line (IC50) even at a concentration of 100 μg mL−1. However, free DOX reached 50% inhibition of growth of the L929 cell at a concentration of 3.81 μg mL−1. Therefore samples B, D and the DOX-loaded sample D show very high cell viability (>70%) against L929 cells, even at high concentrations (100 μg mL−1), which suggests that they may be suitable for drug delivery systems. The MTT assay of these samples was also investigated for the HeLa cell line. The IC50 values of free DOX and the DOX-loaded sample on the HeLa cell line were 3.70 and 14.84 μg mL−1, respectively. The similar IC50 values obtained for free DOX in both the L929 and HeLa cell lines indicates the comparable effect on both cell lines. However, the DOX-loaded sample D shows a greater cytotoxicity against the HeLa cell line (IC50 = 14.84 μg mL−1) compared with the L929 cell line. The high cytotoxicity of the FA-conjugated, DOX-loaded PMs towards the HeLa cell line demonstrate their potentially effective performance in cancer treatment.Cellular uptake
The cellular uptake of the DOX-loaded PMs was studied by confocal laser scanning microscopy for both the L929 and HeLa cell lines using the emergence of the red fluorescence of DOX.47 To examine the targeting ability of the PMs, FA-conjugated DOX-loaded PMs were incubated with both the HeLa and L929 cell lines. In contrast to the L929 cell line (Fig. S5†), a high level of red fluorescence was seen in the HeLa cell lines (Fig. 8) after just 1 h of incubation. The bright fluorescence of the DOX-loaded PMs on the HeLa cell line is attributed to the presence of an FA receptor that directs the FA-conjugated PM specifically towards the HeLa cells.9,29,48 The very high cytotoxicity of the DOX-loaded PMs against HeLa cells compared with L929 cells (Fig. 7) supports these results for cellular uptake. The uptake of DOX-loaded PMs by HeLa cells (Fig. 8) shows red fluorescence mainly in the cytoplasm and partly in the nucleus. The targeting ability of FA-conjugated PMs against FA-unconjugated PMs on HeLa cells is shown in Fig. S6 and S7.† The very high intensity of red fluorescence in HeLa cells (Fig. S6) also clearly shows the targeting ability of FA. | ||
| Fig. 8 Confocal microscopic images of DOX-loaded PMs on HeLa cells (scale bar 40 μm). The nuclei are stained with DAPI. | ||
The appearance of red fluorescence after only 1 h of incubation may be attributed to the partial release of DOX in the acidic environment of the HeLa cell, which then diffuses through the endocytic compartment to the nucleus. However, over a longer period of time the intensity of the red fluorescence increases in the cytoplasm as well as in the nucleus of the cell. This suggests that the uptake of the DOX-loaded PM may follow an endocytic mechanism.22,24
In vitro DOX release
The pH-sensitive release of a drug is crucial in the treatment of cancer. The in vitro DOX release of FA-conjugated samples B and D was studied at 37 °C in PBS (pH 7.4) and acetate buffer (pH 5.0) (Fig. 9). The amount of DOX released was calculated from the UV-visible spectrum (λmax = 482 nm) using the calibration graph of eqn (3). At pH 7.4, only 12% and 13% of DOX were released within 24 h from the DOX-loaded PMs prepared from samples B and D, respectively. This slow release rate at the physiological pH of the bloodstream may decrease the side-effects of the drug. At pH 5.0, however, about 49% and 58% of the DOX was released within 24 h from samples B and D, respectively. The comparatively faster release rate of DOX at the physiological pH (5.0) of cancer cells illustrates the potentially enhanced efficiency of cancer treatment. The release rates for DOX show an increasing trend at low pH (acidic media) compared with high pH (neutral media); this is attributed to two important factors. The first factor is that the greater solubility of DOX in acidic media (pH 5.0) allows the DOX molecule to diffuse from the PM more quickly and efficiently than at the physiological pH (7.4) of the bloodstream.49 The second factor is the hydrogen bonding interaction of the primary amine (–NH2) and the carboxyl (–COOH) groups of the DOX molecule with the carbonyl groups of the PCL segment.22,50 There is effective hydrogen bonding among these groups at the physiological pH (7.4) of the bloodstream. This hydrogen bonding prohibited the DOX molecule from diffusing through the PM, which is reflected in its release profile (Fig. 8). However, at the physiological pH (5.0) of cancer cells, the –NH2 and –COOH groups of the DOX molecule are protonated, which leads to weakening of the hydrogen bonding interactions between DOX and the polymer. This weakening effect allows the DOX molecules to diffuse easily from the PM to the outer medium.51 Samples B and D displayed different release rates at pH 5 than at physiological pH. At pH 5, a lower release rate of sample B compared with sample D can be explained by considering the proportion of the hydrophobic segment (PCL) in the samples. The PCL content is higher in sample B (about 58%, Table 1) compared with sample D (about 43%, Table 1). The hydrophobicity of DOX helps to create hydrophobic–hydrophobic interactions that may be responsible for the lower rate of DOX release.
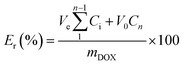 | (3) |
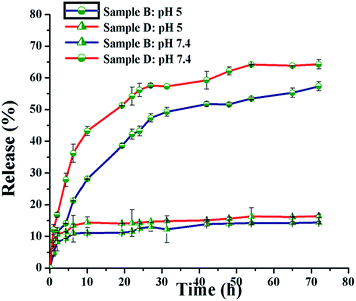 | ||
| Fig. 9 In vitro release profile of DOX from DOX-loaded PMs at pH 5.0 and 7 based on eqn (3). | ||
Conclusion
Branched PE-PCL-b-PEC has been successfully synthesized by ring-opening polymerization. FA is attached at its edge by a DCC coupling reaction. The shift of the gel-permeation chromatogram towards a lower elution time and the appearance of a glass transition temperature (seen on DSC) at about 22 °C (the signature of PEC) confirmed the formation of the block copolymer. The NMR signatures also established its chemical structure. The FA-coupled block copolymer forms a biocompatible and biodegradable PM in aqueous media. The spherical micelles with an average size of 80 nm were confirmed by HRTEM, DLS and AFM. The low CMC value (4.21 × 10−3 mg mL−1, sample A) of the block copolymer PMs (evaluated by fluorescence spectrometry) suggests their potential application in drug delivery systems. PMs produced from branched polymers can encapsulate DOX molecules and show a maximum DLC of 14.7% (sample A). The decrease in the DLC from sample A to sample D is clearly explained by considering the proportion of the hydrophobic (PCL) part in the different block copolymers. The very high degree of cell viability obtained when using the PMs, as evaluated from an MTT assay, is encouraging for its in vivo use in drug delivery systems. The very high cytotoxic effect of FA-conjugated DOX-loaded PM on the HeLa cell line compared with the L929 cell line shows the selective interaction of the PMs on HeLa cells. The cell uptake study also showed a bright fluorescence with HeLa cell lines compared with L929 cell lines after an equivalent incubation time. These two results demonstrate the selective targeting ability of FA-conjugated PM on the HeLa cell line. A high release rate of DOX from the PMs at the physiological pH (pH 5.0) of cancer cells was successfully explained by taking into consideration the better solubility of DOX in acidic media. The high release rate of DOX from the PMs at the physiological pH of cancer cells is responsible for the rapid treatment of cancer by supplying the required amount of DOX. In summary, the FA-conjugated PMs prepared in this work are promising nanocarriers for the targeted delivery of DOX or similar drug molecules to cancer cells.Acknowledgements
SP is grateful to the UGC, New Delhi for sponsoring a fellowship. We acknowledge Mithu Baidya and Somnath Maji for their valuable suggestions.References
- Y. Q. Yang, B. Zhao, Z. D. Li, W. J. Lin, C. Y. Zhang, X. D. Guo, J. F. Wang and L. J. Zhang, Acta Biomater., 2013, 9, 7679–7690 CrossRef CAS PubMed.
- J. I. Chen and W. C. Wu, Macromol. Biosci., 2013, 13, 623–632 CrossRef CAS PubMed.
- A. Kelsch, S. Tomcin, K. Rausch, M. Barz, V. Mailander, M. Schmidt, K. Landfester and R. Zentel, Biomacromolecules, 2012, 13, 4179–4187 CrossRef CAS PubMed.
- X.-Z. Zhang, Y.-Y. Li, H. Cheng, G.-C. Kim, S.-X. Cheng and R.-X. Zhuo, Biomacromolecules, 2006, 7, 2956–2960 CrossRef PubMed.
- Y. B. Patil, U. S. Toti, A. Khdair, L. Ma and J. Panyam, Biomaterials, 2009, 30, 859–866 CrossRef CAS PubMed.
- Y. Li, H. J. Heo, G. H. Gao, S. W. Kang, C. T. Huynh, M. S. Kim, J. W. Lee, J. H. Lee and D. S. Lee, Polymer, 2011, 52, 3304–3310 CrossRef CAS.
- F. Zhu, Q. Yang, Y. Zhuang, Y. Zhang, Z. Shao, B. Gong and Y.-M. Shen, Polymer, 2014, 55, 2977–2985 CrossRef CAS.
- L. Yu, G. Chang, H. Zhang and J. Ding, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 1122–1133 CrossRef CAS.
- W. S. Shim, S. W. Kim, E. K. Choi, H. J. Park, J. S. Kim and D. S. Lee, Macromol. Biosci., 2006, 6, 179–186 CrossRef CAS PubMed.
- Y. Cheng, J. Hao, L. A. Lee, M. C. Biewer, Q. Wang and M. C. Stefan, Biomacromolecules, 2012, 13, 2163–2173 CrossRef CAS PubMed.
- X. Wang, Y. Wang, L. Li, Z. Gu and X. Yu, RSC Adv., 2014, 4, 24810–24815 RSC.
- Y. Hao, J. He, S. Li, J. Liu, M. Zhang and P. Ni, J. Mater. Chem. B, 2014, 2, 4237–4249 RSC.
- J. Chen and M. Liu, RSC Adv., 2014, 4, 9684–9692 RSC.
- W. Lin, S. Nie, Q. Zhong, Y. Yang, C. Cai, J. Wang and L. Zhang, J. Mater. Chem. B, 2014, 2, 4008–4020 RSC.
- J. X. Zhang, L. Y. Qiu, Y. Jin and K. J. Zhu, J. Biomed. Mater. Res., Part A, 2006, 76, 773–780 CrossRef PubMed.
- A. K. S. Esfandiar Behravesh, S. Jo and A. G. Mikos, Biomacromolecules, 2002, 3, 153–158 CrossRef.
- H. Feng and C.-M. Dong, Biomacromolecules, 2006, 7, 3069–3075 CrossRef CAS PubMed.
- Y. Wang, X. Wang, L. Li, Z. Gu and X. Yu, RSC Adv., 2014, 4, 24810–24815 RSC.
- M. Ji Hwang, J. Myung Suh, Y. Han Bae, S. Wan Kim and B. Jeong, Biomacromolecules, 2005, 8, 885–890 CrossRef PubMed.
- S. D. Vincent Pourcelle, M. Garinot, V. Préat and J. Marchand-Brynaert, Biomacromolecules, 2007, 8, 3977–3983 CrossRef PubMed.
- Y. W. Ying Wang, J. Xiang and K. Yao, Biomacromolecules, 2010, 11, 3531–3538 CrossRef PubMed.
- L. Chang, L. Deng, W. Wang, Z. Lv, F. Hu, A. Dong and J. Zhang, Biomacromolecules, 2012, 13, 3301–3310 CrossRef CAS PubMed.
- Q. L. Liu, R. Zhu, Z. Qian, X. Guan, W. Yu, L. Yang, M. Jiang, X. Liu and B. Liu, Int. J. Pharm., 2012, 430, 350–358 CrossRef CAS PubMed.
- Y. Jin, L. Song, Y. Su, L. Zhu, Y. Pang, F. Qiu, G. Tong, D. Yan, B. Zhu and X. Zhu, Biomacromolecules, 2011, 12, 3460–3468 CrossRef CAS PubMed.
- D. Wang, Y. Su, C. Jin, B. Zhu, Y. Pang, L. Zhu, J. Liu, C. Tu, D. Yan and X. Zhu, Biomacromolecules, 2011, 12, 1370–1379 CrossRef CAS PubMed.
- L. -Y. Li, W. -D. He, J. Li, B. -Y. Zhang, T.-T. Pan, X.-L. Sun and Z.-L. Ding, Biomacromolecules, 2010, 11, 1882–1890 CrossRef CAS PubMed.
- J. Sun, X. Chen, J. Guo, Q. Shi, Z. Xie and X. Jing, Polymer, 2009, 50, 455–461 CrossRef CAS.
- U. G. Prateek Singh, A. Asthana and N. K. Jain, Bioconjugate Chem., 2008, 19, 2239–2252 CrossRef PubMed.
- S. Chen, X. -Z. Zhang, S. -X. Cheng, R.-X. Zhuo and Z.-W. Gu, Biomacromolecules, 2008, 9, 2578–2585 CrossRef CAS PubMed.
- L. Yu, T. Ci, S. Zhou, W. Zeng and J. Ding, Biomater. Sci., 2013, 1, 411–420 RSC.
- Z. X. Wenjun Du, A. M. Nyström, K. Zhang, J. R. Leonard and K. L. Wooley, Bioconjugate Chem., 2008, 19, 2492–2498 CrossRef PubMed.
- M. Prabaharan, J. J. Grailer, S. Pilla, D. A. Steeber and S. Gong, Macromol. Biosci., 2009, 9, 515–524 CrossRef CAS PubMed.
- Y. Zhou and D. Yan, Chem. Commun., 2009, 1172–1188 RSC.
- J. Szebeni, Toxicology, 2005, 216, 106–121 CrossRef CAS PubMed.
- T. Ishida, M. Harada, X. Y. Wang, M. Ichihara, K. Irimura and H. Kiwada, J. Controlled Release, 2005, 105, 305–317 CrossRef CAS PubMed.
- G. Pasut and F. M. Veronese, Prog. Polym. Sci., 2007, 32, 933–961 CrossRef CAS.
- F. Unger, U. Westedt, P. Hanefeld, R. Wombacher, S. Zimmermann, A. Greiner, M. Ausborn and T. Kissel, J. Controlled Release, 2007, 117, 312–321 CrossRef CAS PubMed.
- F. N. Murat Acemoglu, S. Bantle and G. H. Stoll, J. Controlled Release, 1997, 49, 263–276 CrossRef.
- S. Panja, B. Saha, S. K. Ghosh and S. Chattopadhyay, Langmuir, 2013, 29, 12530–12540 CrossRef CAS PubMed.
- S. H. Park, B. G. Choi, M. K. Joo, D. Keun Han, Y. Soo Sohn and B. Jeong, Macromolecules, 2008, 41, 6486–6492 CrossRef CAS.
- Y. T. M. Licciardi, N. C. Billingham and S. P. Armes, Biomacromolecules, 2005, 6, 1085–1096 CrossRef PubMed.
- P. Chan, M. Kurisawa, J. E. Chung and Y. Y. Yang, Biomaterials, 2007, 28, 540–549 CrossRef CAS PubMed.
- A. Sulistio, J. Lowenthal, A. Blencowe, M. N. Bongiovanni, L. Ong, S. L. Gras, X. Zhang and G. G. Qiao, Biomacromolecules, 2011, 12, 3469–3477 CrossRef CAS PubMed.
- Y. Mi, Y. Liu and S. S. Feng, Biomaterials, 2011, 32, 4058–4066 CrossRef CAS PubMed.
- X. Hu, X. Chen, S. Liu, Q. Shi and X. Jing, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 1852–1861 CrossRef CAS.
- G. Gaucher, M. H. Dufresne, V. P. Sant, N. Kang, D. Maysinger and J. C. Leroux, J. Controlled Release, 2005, 109, 169–188 CrossRef CAS PubMed.
- C. Zhang, S. Jin, S. Li, X. Xue, J. Liu, Y. Huang, Y. Jiang, W. Q. Chen, G. Zou and X. J. Liang, ACS Appl. Mater. Interfaces, 2014, 6, 5212–5220 CAS.
- T. Chen, S. Xu, T. Zhao, L. Zhu, D. Wei, Y. Li, H. Zhang and C. Zhao, ACS Appl. Mater. Interfaces, 2012, 4, 5766–5774 CAS.
- J. Yue, R. Wang, S. Liu, S. Wu, Z. Xie, Y. Huang and X. Jing, Soft Matter, 2012, 8, 7426–7435 RSC.
- Z. Xu, D. Wang, M. Guan, X. Liu, Y. Yang, D. Wei, C. Zhao and H. Zhang, ACS Appl. Mater. Interfaces, 2012, 4, 3424–3431 CAS.
- J. Fan, F. Zeng, S. Wu and X. Wang, Biomacromolecules, 2012, 13, 4126–4137 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra08351b |
| This journal is © The Royal Society of Chemistry 2014 |