Mussel-inspired ultrathin film on oxidized Ti–6Al–4V surface for enhanced BMSC activities and antibacterial capability
Ziyuan Wanga,
Malcolm Xing*ab and
Olanrewaju Ojo*a
aDepartment of Mechanical Engineering, University of Manitoba, Winnipeg, Manitoba, Canada. E-mail: Malcolm.xing@umanitoba.ca; Olanrewaju.Ojo@umanitoba.ca
bManitoba Institute of Child Health, Winnipeg, Manitoba, Canada
First published on 2nd October 2014
Abstract
To improve the biocompatibility and antibacterial capability of thermally oxidized Ti–6Al–4V, an ultrathin alginate/chitosan film that contains nano-silver was constructed through mussel-inspired poly(dopamine). The hybrid structure was successfully fabricated on the Ti–6Al–4V surface without deterioration of the rough surface induced by thermal oxidation (TO). The hybrid surface was characterized by the use of contact angle goniometry (CAG), atomic force microscopy (AFM), scanning electron microscopy (SEM), X-ray photoelectron spectrometry (XPS), and energy dispersive spectrometry (EDS). Bone marrow stem cell (BMSC) viability, morphology and proliferation tests showed a significant increase due to the biomimetic chitosan/alginate film on Ti–6Al–4V. Furthermore, nano-silver particles incorporated into the chitosan/alginate film inhibited the growth of Escherichia coli (E. coil) and Staphylococcus aureus (S. aureus) without jeopardizing the improved BMSC viability, morphology and proliferation. More importantly, the expression of alkaline phosphatase (ALP) was significantly up-regulated after BMSCs were cultured on the Ti–6Al–4V-based hybrid structure. Overall, this study presents a promising strategy to improve biocompatibility and antibacterial capability of thermally oxidized Ti–6Al–4V alloy.
Introduction
Various surface modification techniques have been employed to alter the surface characteristics of bio-implantable titanium and its alloys. These include traditional techniques such as thermal oxidation (TO), chemical treatment and sandblasting, and recently emerged techniques like layer-by-layer (LBL) self-assembly of polyelectrolytes, nano-patterning and biomolecule immobilization, etc.1–7 Among traditional techniques, TO can produce a nodular surface topography that consists of nano- to micron-scale fine oxide particles.8 Both in vitro and in vivo studies have demonstrated that this nodular topography is advantageous in bone–implant integration.1,9,10 However, the bio-inert nature of thermally oxidized titanium and its alloys makes them unable to mimic the extracellular microenvironment, which is essential in regulating the implant–cell interactions.11 Therefore, further modification of thermally oxidized titanium and its alloys is very necessary.Besides inadequate biocompatibility, bacterial contamination also largely compromises the long-term success of implants.12 The biofilm formed at the bone–implant interface is highly resistant to host defense and accounts for a high percentage of implant failure. One of the most promising strategies to overcome this situation is the incorporation of nano-silver into implants. It has been demonstrated that at suitable dose, nano-silver exhibits wide antibacterial spectrum, low cytotoxicity and long-term antibacterial characteristics.13
Recently, the LBL self-assembly of polyelectrolytes has gained increasing interest. The films formed by electrostatic force attraction are ideal platforms to mimic the extracellular microenvironment.14 Assembly of different polyelectrolytes such as chitosan/alginate, PLL/DNA and chitosan/gelatin have been carried out to regulate implant–cell interactions.15–19 Meanwhile, the LBL film can also function as a carrier to load bone-related chemicals like fibronectin, BMP 2, etc.20 However, this technique has not been reported to improve the biocompatibility and antibacterial capability of thermally oxidized Ti–6Al–4V surface.
Aims to improve the biocompatibility and antibacterial capability of thermally oxidized Ti–6Al–4V, we herein fabricated an ultrathin alginate/chitosan surface film that contains nano-silver through poly(dopamine), which exhibits a comparable structure to the adhesive proteins secreted by mussels (Scheme 1). In this study, we first monitored the alginate/chitosan film fabrication process and selected the optimal alginate/chitosan pair number to be constructed onto the thermally oxidized Ti–6Al–4V surface. Then we incorporated nano-silver into the selected alginate/chitosan film and characterized the topographical properties of different samples. Finally, the in vitro performance of the Ti–6Al–4V-based hybrid structure was evaluated through BMSC viability, morphology, proliferation and differentiation assays.
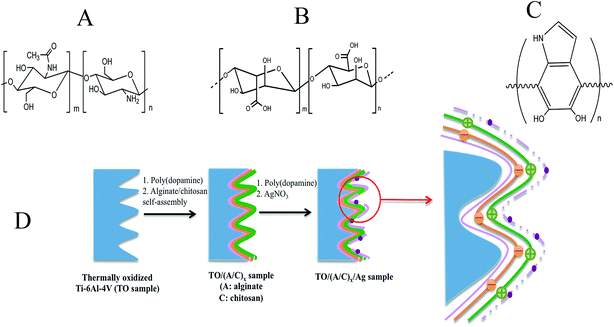 | ||
| Scheme 1 The molecular structure of (A) chitosan, (B) alginate, (C) poly(dopamine) and (D) the construction process of the mussel-inspired film onto thermally oxidized Ti–6Al–4V surface. | ||
Materials and methods
Materials
A Ti–6Al–4V (ASTM F-1472) round bar that was 12.7 mm in diameter was purchased from Titanium Industries, Inc. (QB, Canada). Medium molecular weight chitosan (75–85% deacetylated) and alginic acid sodium salt (viscosity of 2% solution at 25 °C–250 cps) were purchased from Sigma-Aldrich Chemical Co. (MO, USA). Dopamine hydrochloride was obtained from Alfa Aesar (MA, USA). Other chemicals were purchased from Fisher Scientific (ON, Canada). Mouse BMSCs were purchased from American Type Culture Collection (MD, USA). As received Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS) and related cell culture reagents were purchased from Gibco (ON, Canada) and used without further treatment. Live/Dead® viability assay kit, TRIzol and Oligo dT were obtained from Invitrogen (CA, USA). methylthiazoltetrazolium bromide (MTT) cell proliferation assay kits were purchased from Biotium Inc. (CA, USA). All chemicals were used as-received unless otherwise specified.Methods
A JEOL 5900 scanning electron microscope was employed to characterize the surface topographies of Ti–6Al–4V samples. Additionally, an X-ray photoelectron spectroscopy analysis was carried out with an Axis DLD Ultra X-ray photoelectron spectrometer with an Al Kα (1486.6 eV) monochromatic source at base pressures less than 10−8 Torr and a perpendicular take-off angle. The energy shift due to the surface charge was corrected with C 1s that had a binding energy of 285 eV.
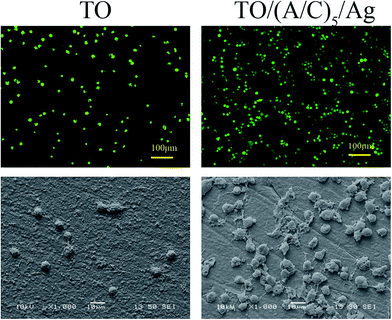
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per cm2. After 1 day of cell culture, the samples were washed twice with PBS and transferred to a new culture flask. For Live & Dead assay, each sample was pipetted with 100 μl reagents and incubated for 30 min at room temperature in the dark. After a subsequent washing step with PBS, cell viability was examined by the use of an Olympus X51 fluorescence microscopy. For cell morphology observation, Ti–6Al–4V samples were immersed in the mixture of 2.5% glutaraldehyde and 4% paraformaldehyde solution for 4 h at 4 °C, followed by washing twice with PBS for 10 min. To dehydrate the BMSCs, all samples were alternatively treated twice with 50%, 75%, 95%, and 100% ethanol for 15 min. Finally, the samples were coated with platinum by using a technosyn cold cathode luminescence system and examined by a JEOL 5900 SEM.
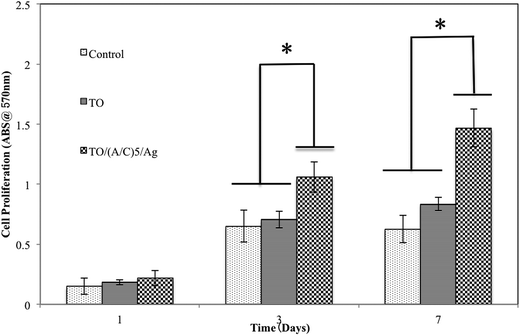
000 cells per cm2. After 1 day of cell culture, the samples were washed twice with PBS and transferred to a new culture flask. For Live & Dead assay, each sample was pipetted with 100 μl reagents and incubated for 30 min at room temperature in the dark. After a subsequent washing step with PBS, cell viability was examined by the use of an Olympus X51 fluorescence microscopy. For cell morphology observation, Ti–6Al–4V samples were immersed in the mixture of 2.5% glutaraldehyde and 4% paraformaldehyde solution for 4 h at 4 °C, followed by washing twice with PBS for 10 min. To dehydrate the BMSCs, all samples were alternatively treated twice with 50%, 75%, 95%, and 100% ethanol for 15 min. Finally, the samples were coated with platinum by using a technosyn cold cathode luminescence system and examined by a JEOL 5900 SEM.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per cm2 were seeded on the samples. Then 1 ml DMEM medium was added to each well. After 1, 3 and 7 days of culturing, each well was added with 100 μl MTT (5 mg ml−1) and incubated at 37 °C for 4 h. Then the samples were transferred to a new 24-well plate and 0.5 ml dimethyl sulfoxide (DMSO) was added to dissolve the formazan crystals. After 15 min, 200 μl DMSO was extracted to a new 96 well-plate. Finally, the absorbance was recorded at a wavelength of 570 nm.
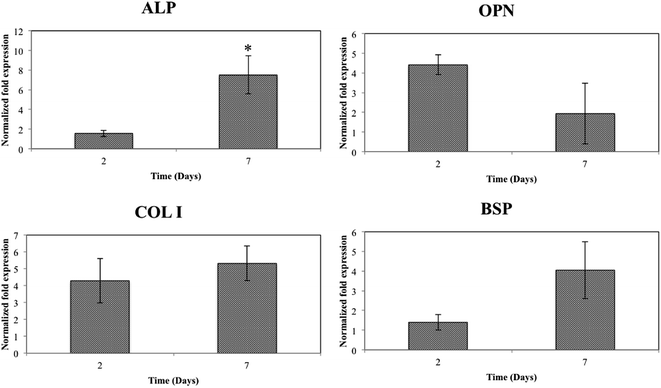
000 cells per cm2 were seeded on the samples. Then 1 ml DMEM medium was added to each well. After 1, 3 and 7 days of culturing, each well was added with 100 μl MTT (5 mg ml−1) and incubated at 37 °C for 4 h. Then the samples were transferred to a new 24-well plate and 0.5 ml dimethyl sulfoxide (DMSO) was added to dissolve the formazan crystals. After 15 min, 200 μl DMSO was extracted to a new 96 well-plate. Finally, the absorbance was recorded at a wavelength of 570 nm.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per cm2 and collected after 2 and 7 days of osteogenic induction. The samples without osteogenic induction (day 0) were used as control. The total ribonucleic acid (RNA) was extracted by using TRIzol in accordance with the supplier instructions. Then 1 μg RNA sample was reversely transcribed for standard cDNA synthesis. The q-RT-PCR was conducted by an SYBER Green assay (Applied Biosystems, USA). Amplification program was started with an initial denaturation at 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s and 60 °C for 1 min. The five gene premiers used in this study were (form 5′–3′): ALP-F: CTC CAA AAG CTC AAC ACC AAT G, ALP-R: ATT TGT CCA TCT CCA GCC G; BSP-F: CCA CAC TTT CCA CAC TCT CG, BSP-R: CGT CGC TTT CCT TCA CTT TTG; COL I–F: AAC AGT CGC TTC ACC TAC AG, COL I-R: AAT GTC CAA GGG AGC CAC; OPN-F: CTA CGA CCA TGA GAT TGG CAG, OPN-R: CAT GTG GCT ATA GGA TCT GGG; GAPDH-F: AGG TCG GTG TGA ACG GAT TTG, GAPDH-R: TGT AGA CCA TGT AGT TGA GGT CA. The relative expressions of genes were determined by the 2−ΔΔCt method. GAPDH was selected as a housekeeping gene to normalize the expression levels of the target genes.
000 cells per cm2 and collected after 2 and 7 days of osteogenic induction. The samples without osteogenic induction (day 0) were used as control. The total ribonucleic acid (RNA) was extracted by using TRIzol in accordance with the supplier instructions. Then 1 μg RNA sample was reversely transcribed for standard cDNA synthesis. The q-RT-PCR was conducted by an SYBER Green assay (Applied Biosystems, USA). Amplification program was started with an initial denaturation at 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s and 60 °C for 1 min. The five gene premiers used in this study were (form 5′–3′): ALP-F: CTC CAA AAG CTC AAC ACC AAT G, ALP-R: ATT TGT CCA TCT CCA GCC G; BSP-F: CCA CAC TTT CCA CAC TCT CG, BSP-R: CGT CGC TTT CCT TCA CTT TTG; COL I–F: AAC AGT CGC TTC ACC TAC AG, COL I-R: AAT GTC CAA GGG AGC CAC; OPN-F: CTA CGA CCA TGA GAT TGG CAG, OPN-R: CAT GTG GCT ATA GGA TCT GGG; GAPDH-F: AGG TCG GTG TGA ACG GAT TTG, GAPDH-R: TGT AGA CCA TGT AGT TGA GGT CA. The relative expressions of genes were determined by the 2−ΔΔCt method. GAPDH was selected as a housekeeping gene to normalize the expression levels of the target genes.Results and discussion
Characterization of the alginate/chitosan film construction process
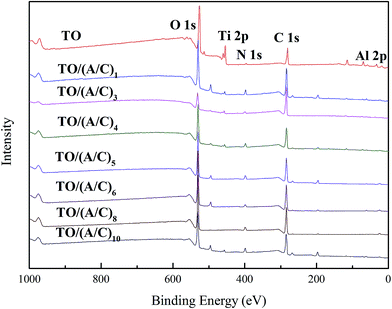
This part reports the construction process of the alginate/chitosan film on thermally oxidized Ti–6Al–4V samples. An XPS was used to investigate the surface chemistry change during the alginate/chitosan LBL assembly process. All of the spectra obtained indicate the major elements of Ti, Al, C, N and O (Fig. 1). A gradual decrease in the intensity of Ti 2p and Al 2p was observed with an increasing number of alginate/chitosan pairs (data not shown). The N 1s high resolution spectra (Fig. 2) demonstrate the presence of amine groups at binding energies about 398.1, with respect to the use of chitosan.21 The amide peaks at about 401.3 eV were possibly caused by atmospheric contamination from thermal oxidation and the partial N-deacetylation of chitin.22 Meanwhile, the gradual increase in the intensity of amine group implies that there was a sustained deposition of chitosan onto the TO sample surface. Taken together, the X-ray spectrometry analysis confirms the layerwise deposition of alginate/chitosan onto the thermally oxidized Ti–6Al–4V surface.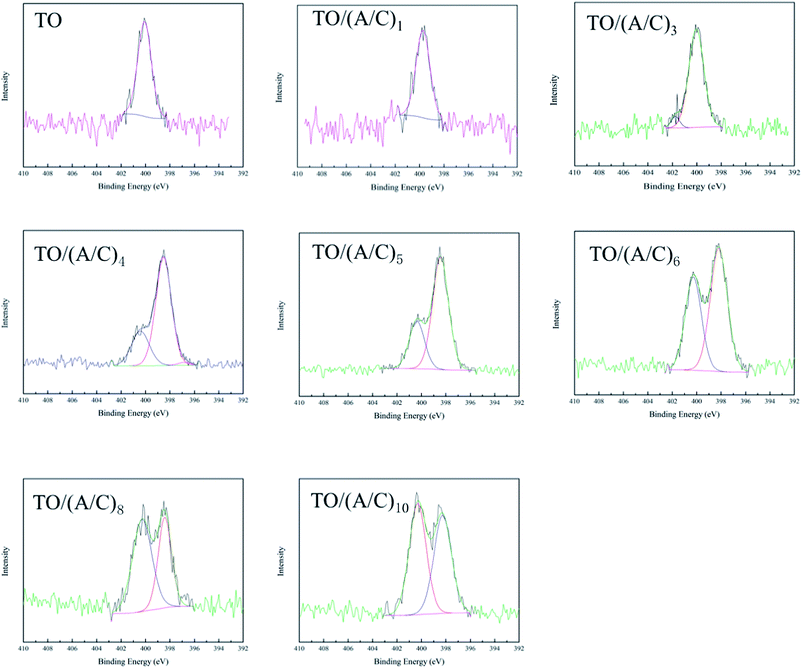 | ||
| Fig. 2 N 1s high resolution spectra of Ti–6Al–4V after different treatments (A: alginate; C: chitosan). | ||
The water contact angle measurement was carried out to monitor the variation of wettability during the alginate/chitosan film construction process (Fig. 3). An alternative change in the water contact angle was observed after the deposition of each alginate and chitosan single layer. This phenomenon is consistent with previous study and indicates that the TO samples were fully covered by either alginate or chitosan as the outmost layer.21 Interestingly, J. H. Park et al. observed and speculated that the incomplete coverage of chitosan, poly(L-glutamic acid) and poly(L-lysine) monolayers on titanium surface is due to the 0.15 M NaCl used to dissolve these polyelectrolytes, which results in the preferential attachment of Na+ onto the negatively charged Ti surface.23 However, this phenomenon is not observed in this study. It can be attributed to the PDA used prior to alginate/chitosan LBL self-assembly. The strong adhesive properties of the PDA precursor ensured the uniform deposition of the first alginate layer onto the TO sample, which in turn provided a desirable negatively charged surface for the deposition of the first chitosan layer. Accordingly, a well-organized LBL film can be formed after the alternative deposition of alginate and chitosan.
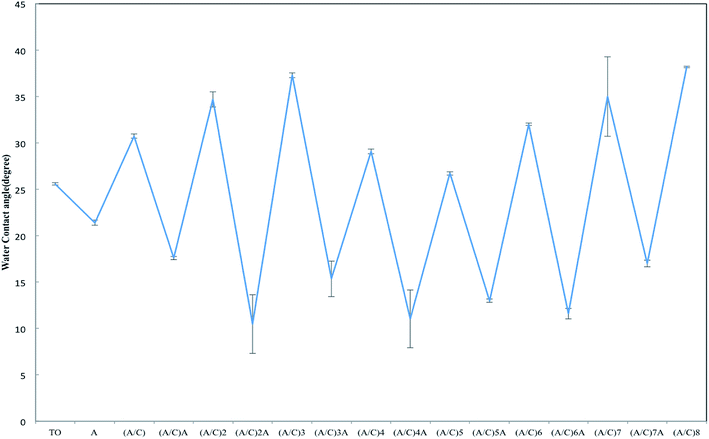 | ||
| Fig. 3 Water contact angle measurement during the alginate/chitosan film construction process (A: alginate; C: chitosan). | ||
AFM was employed to monitor the surface roughness and topography changes during the alginate/chitosan film construction process. Fig. 4 shows that the initial layerwise build-up did not significantly alter the surface topography produced by TO. However, the original oxide particles became larger in TO/(A/C)6 to TO/(A/C)10 samples. This phenomenon clearly indicates that the surface topography induced by TO had been altered after six pairs of alginate/chitosan LBL assembly. The topography change was further quantified by a roughness measurement. As shown in Fig. 5, the average surface roughness values were stable around 80 nm in TO/(A/C) to TO/(A/C)5 samples. However, the surface roughness increased to 120.03 ± 22.21 nm on TO/(A/C)6 sample, and finally reached a value of 211.50 ± 22.95 nm on TO/(A/C)10 sample. Theoretically, the spin-assisted coating would produce uniform surface layers on a flat surface, even to fabricate one single layer. However, when the surface is not flat, as shown in the current study, the result becomes more complex. It is speculated that the sudden increase of roughness was possibly caused by the rough surface, which cannot be uniformly covered by chitosan and alginate. As the increase of layer number, the surface may become rougher than conventionally dip-coated flat surfaces. Further investigation may be conducted to reveal this phenomenon. Taken together, the film made of five pairs of alginate/chitosan are preferred as an “ultrathin” film for further study because it was able to fully cover the TO sample surface, but did not significantly alter its surface roughness and topography. Further studies were, therefore, centered on the TO/(A/C)5 sample (Fig. 6).
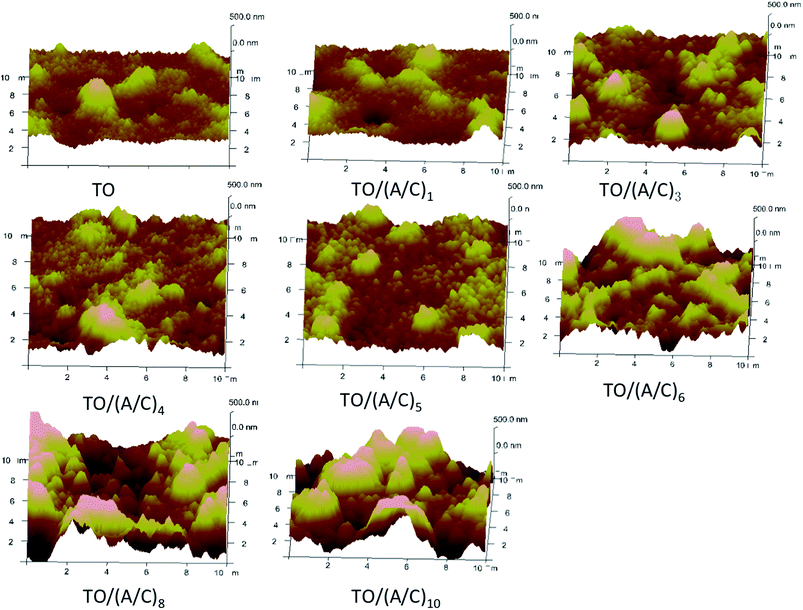 | ||
| Fig. 4 Surface topography change of thermally oxidized Ti–6Al–4V samples during alginate/chitosan film build-up process (A: alginate; C: chitosan. AFM scan area: 10 × 10 μm2, z-axis height: 500 nm). | ||
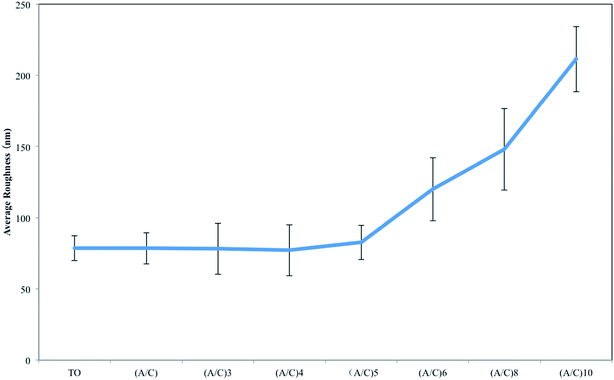 | ||
| Fig. 5 Surface roughness measurement of thermally oxidized Ti–6Al–4V samples with different number of alginate/chitosan pairs (A: alginate; C: chitosan). | ||
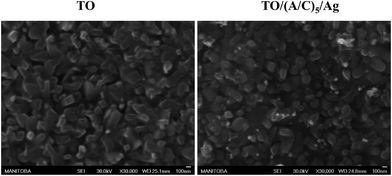
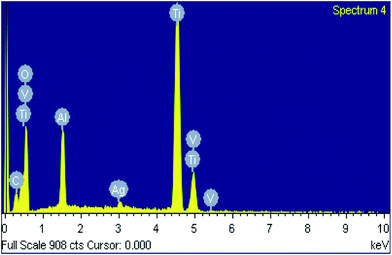
Scanning electron microscope was used to characterize the topography of modified Ti–6Al–4V samples. The TO sample surface was consisted of numerous fine oxide particles. The TO/(A/C)5 sample exhibited a comparable topography to the TO sample, but with additional nano-scale shinny particles (30–80 nm). Energy dispersive spectrometry analysis demonstrates that these shiny particles appeared to be Ag (Fig. 7). Therefore, it is demonstrated that the expected hybrid thin film has been successfully fabricated on the TO sample.
In vitro evaluation
Conclusion
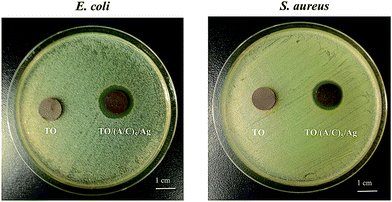
In this study, an alginate/chitosan ultrathin film that contains nano-silver particles was successfully constructed on thermally oxidized Ti–6Al–4V surface by the use of mussel-inspired dopamine. The hybrid structure completely altered the surface chemistry of Ti–6Al–4V without deteriorating the rough surface layer induced by TO. Improvements in the BMSC viability, morphology, proliferation and demonstrated antibacterial capability have been observed in vitro. More importantly, BMSCs cultured onto the Ti–6Al–4V-based hybrid structure exhibited significantly higher ALP expression compared to the controls at 7 days' osteogenic induction. Overall, the combination of the surface characterizations and in vitro assays demonstrates that the hybrid structure made of alginate/chitosan ultrathin film and nano-silver, developed in this work, presents functionality in enhancing the BMSC viability, proliferation and initial ALP expression, as well as antibacterial capability of thermally oxidized Ti–6Al–4V alloy.Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Conflict of interest
The authors declare no competing financial interest.Acknowledgements
Financial supports from the NSERC of Canada, the Manitoba Health Research Council and the Manitoba Institute of Child Health are gratefully acknowledged.References
- L. Saldana, N. Vilaboa, G. Valles, J. Gonzalez-Cabrero and L. Munuera, Osteoblast response to thermally oxidized Ti6Al4V alloy, J. Biomed. Mater. Res., Part A, 2005, 73(1), 97–107 CrossRef CAS PubMed
.
- S.-H. An, T. Matsumoto, H. Miyajima, J.-I. Sasaki, R. Narayanan and K.-H. Kim, Surface characterization of alkali- and heat-treated Ti with or without prior acid etching, Appl. Surf. Sci., 2012, 258(10), 4377–4382 CrossRef CAS PubMed
.
- A. E. Daw, H. A. Kazi, J. S. Colombo, W. G. Rowe, D. W. Williams, R. J. Waddington, D. W. Thomas and R. Moseley, Differential cellular and microbial responses to nano-/micron-scale titanium surface roughness induced by hydrogen peroxide treatment, J. Biomater. Appl., 2013, 28(1), 144–160 CrossRef CAS PubMed
.
- S. G. Yan, J. Zhang, Q. S. Tu, J. H. Ye, E. Luo, M. Schuler, M. S. Kim, T. Griffin, J. Zhao, X. J. Duan, D. J. Cochran, D. Murray, P. S. Yang and J. Chen, Enhanced osseointegration of titanium implant through the local delivery of transcription factor SATB2, Biomaterials, 2011, 32(33), 8676–8683 CrossRef CAS PubMed
.
- J. Fiedler, B. Özdemir, J. Bartholomä, A. Plettl, R. E. Brenner and P. Ziemann, The effect of substrate surface nanotopography on the behavior of multipotent mesenchymal stromal cells and osteoblasts, Biomaterials, 2013, 34(35), 8851–8859 CrossRef CAS PubMed
.
- L. Ge, Q. Li, Y. Huang, S. Yang, S. Bu, J. Ouyang, W. Zhong, Z. Liu and M. Xing, Polydopamine-coated paper-stack nanofibrous membranes enhancing adipose stem cells' adhesion and osteogenic differentiation, J. Mater. Chem. B, 2014, 2, 6917–6923 RSC
.
- J. Chen, X. Qiu, L. Wang, W. Zhong, J. Kong and M. M. Xing, Free-Standing Cell Sheet Assembled with Ultrathin Extracellular Matrix as an Innovative Approach for Biomimetic Tissues, Adv. Funct. Mater., 2013, 24(15), 2216–2223 CrossRef
.
- S. Kumar, T. Narayanan, S. Raman and S. Seshadri, Thermal oxidation of CP–Ti: evaluation of characteristics and corrosion resistance as a function of treatment time, Mater. Sci. Eng., C, 2009, 29(6), 1942–1949 CrossRef CAS PubMed
.
- M. Alonso, L. Saldana, G. Valles, J. L. González-Carrasco, J. Gonzalez-Cabrero, M. Martınez, E. Gil-Garay and L. Munuera, In vitro corrosion behaviour and osteoblast response of thermally oxidised Ti6Al4V alloy, Biomaterials, 2003, 24(1), 19–26 CrossRef
.
- T. Ueno, N. Tsukimura, M. Yamada and T. Ogawa, Enhanced bone-integration capability of alkali- and heat-treated nanopolymorphic titanium in micro-to-nanoscale hierarchy, Biomaterials, 2011, 32(30), 7297–7308 CrossRef CAS PubMed
.
- M. Lutolf and J. Hubbell, Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering, Nat. Biotechnol., 2005, 23(1), 47–55 CrossRef CAS PubMed
.
- J. Schierholz and J. Beuth, Implant infections: a haven for opportunistic bacteria, J. Hosp. Infect., 2001, 49(2), 87–93 CrossRef CAS PubMed
.
- L. Ge, Q. Li, M. Wang, J. Ouyang, X. Li and M. Xing, Nanosilver particles in medical applications: synthesis, performance, and toxicity, Int. J. Nanomed., 2014, 9, 2399–2407 Search PubMed
.
- Y. Hu, K. Cai, Z. Luo, Y. Zhang, L. Li, M. Lai, Y. Hou, Y. Huang, J. Li and X. Ding, Regulation of the differentiation of mesenchymal stem cells in vitro and osteogenesis in vivo by microenvironmental modification of titanium alloy surfaces, Biomaterials, 2012, 33(13), 3515–3528 CrossRef CAS PubMed
.
- W. Gao, B. Feng, X. Lu, J. Wang, S. Qu and J. Weng, Characterization and cell behavior of titanium surfaces with PLL/DNA modification via a layer-by-layer technique, J. Biomed. Mater. Res., Part A, 2012, 100(8), 2176–2185 CrossRef PubMed
.
- T. Jiang, Z. Zhang, Y. Zhou, Y. Liu, Z. Wang, H. Tong, X. Shen and Y. Wang, Surface functionalization of titanium with chitosan/gelatin via electrophoretic deposition: characterization and cell behavior, Biomacromolecules, 2010, 11(5), 1254–1260 CrossRef CAS PubMed
.
- G. Wang, T. Zhao, X. Song, W. Zhong, L. Yu, W. Hua, M. Xing, and X. Qiu, 3-D multicellular tumor spheroid on ultrathin matrices coated single cancer cells provides a tumor microenvironment model to study epithelial-to-mesenchymal transition, Polym. Chem., 10.1039/C4PY01161A.
- K. Cai, A. Rechtenbach, J. Hao, J. Bossert and K. D. Jandt, Polysaccharide–protein surface modification of titanium via a layer-by-layer technique: characterization and cell behaviour aspects, Biomaterials, 2005, 26(30), 5960–5971 CrossRef CAS PubMed
.
- J. Choi, T. Konno, R. Matsuno, M. Takai and K. Ishihara, Surface immobilization of biocompatible phospholipid polymer multilayered hydrogel on titanium alloy, Colloids Surf., B, 2008, 67(2), 216–223 CrossRef CAS PubMed
.
- Y. Hu, K. Cai, Z. Luo, R. Zhang, L. Yang, L. Deng and K. D. Jandt, Surface mediated in situ differentiation of mesenchymal stem cells on gene-functionalized titanium films fabricated by layer-by-layer technique, Biomaterials, 2009, 30(21), 3626–3635 CrossRef CAS PubMed
.
- G. Lawrie, I. Keen, B. Drew, A. Chandler-Temple, L. Rintoul, P. Fredericks and L. Grøndahl, Interactions between alginate and chitosan biopolymers characterized using FTIR and XPS, Biomacromolecules, 2007, 8(8), 2533–2541 CrossRef CAS PubMed
.
- M. Sugimoto, M. Morimoto, H. Sashiwa, H. Saimoto and Y. Shigemasa, Preparation and characterization of water-soluble chitin and chitosan derivatives, Carbohydr. Polym., 1998, 36(1), 49–59 CrossRef CAS
.
- J. H. Park, Z. Schwartz, R. Olivares-Navarrete, B. D. Boyan and R. Tannenbaum, Enhancement of surface wettability via the modification of microtextured titanium implant surfaces with polyelectrolytes, Langmuir: the ACS journal of surfaces and colloids, 2011, 27(10), 5976–5985 CrossRef CAS PubMed
.
- G. Mendonca, D. B. Mendonca, F. J. Aragao and L. F. Cooper, Advancing dental implant surface technology-from micron- to nanotopography, Biomaterials, 2008, 29(28), 3822–3835 CrossRef CAS PubMed
.
- D. S. Benoit, M. P. Schwartz, A. R. Durney and K. S. Anseth, Small functional groups for controlled differentiation of hydrogel-encapsulated human mesenchymal stem cells, Nat. Mater., 2008, 7(10), 816–823 CrossRef CAS PubMed
.
- J. Ivirico, M. Salmerón-Sánchez, J. Ribelles, M. M. Pradas, J. M. Soria, M. E. Gomes, R. Reis and J. Mano, Proliferation and differentiation of goat bone marrow stromal cells in 3D scaffolds with tunable hydrophilicity, J. Biomed. Mater. Res., Part B, 2009, 91(1), 277–286 CrossRef PubMed
.
- J. Chen, B. Zhou, Q. Li, J. Ouyang, J. Kong, W. Zhong and M. Xing, PLLA-PEG-TCH-labeled bioactive molecule nanofibers for tissue engineering, Int. J. Nanomed., 2011, 6, 2533–2542 CAS
.
- J. Chen, M. Shi, P. Liu, A. Ko, W. Zhong, W. Liao and M. Xing, Reducible polyamidoamine-magnetic iron oxide self-assembled nanoparticles for doxorubicin delivery, Biomaterials, 2014, 35, 1240–1248 CrossRef CAS PubMed
.
- J. Shi, J. Ouyang, Q. Li, L. Wang, J. Wu, W. Zhong and M. Xing, Cell-compatible hydrogels based on a multifunctional crosslinker with tunable stiffness for tissue engineering, J. Mater. Chem., 2012, 22, 23952–23962 RSC
.
| This journal is © The Royal Society of Chemistry 2014 |