An ionogel composite including copolymer nanowires for disposable electrochemiluminescent sensor configurations
I. F. Díaz-Ortega,
J. Ballesta-Claver,
M. Cruz Martín,
S. Benítez-Aranda and
L. F. Capitán-Vallvey*
ECsens, Department of Analytical Chemistry, Campus Fuentenueva, Faculty of Sciences, University of Granada, E-18071 Granada, Spain. E-mail: lcapitan@ugr.es
First published on 15th October 2014
Abstract
Aniline derivatives such as luminol and benzidines can be electropolymerized for the preparation of electrochemiluminescent sensors. However, the development of these sensors requires formulations to improve the chemical stability and photoemissive and electrochemical properties. The use of ionic liquids confined in gels (ionogels) can provide a material for electrochemiluminescent sensor preparation and more specifically by means of hybrid organic–inorganic composites which provide flexibility, thermal stability, and ionic transport. By using this configuration, we prepared nanowires of luminescent polymers by electropolymerization in the pores of a hydrophilic silica ionogel with chitosan, offering a biocomposite that can be prepared in disposable screen-printed gold electrodes. The properties of the prepared biocomposite have been characterized by emissive luminescence, cyclic voltammetry, electrochemical quartz crystal microbalance and scanning electron microscopy. The results show that the electrochemiluminescent copolymer grows efficiently as nanowires in the pores of the biocomposite, forming sensing films that produce an intense luminescent blank signal due to the influence of the ionic liquid. Hydrogen peroxide was used as an analyte; it acts as a scavenger, reducing the emissive signal and resulting in a novel electrochemiluminescence configuration for sensing. This configuration broadens the field of electrochemiluminescence sensors due to the biocompatibility of the developed ionogel membrane.
1. Introduction
Electrochemiluminescence (ECL), the generation of light-emitting species by means of strong oxidizing and reducing agents,1 has great potential as an analytical technique for detection in immunoassays, DNA probe assays, sensors and biosensors, mainly in flow and optical fibre formats, high throughput analysis, microfluidic devices and chromatographic and electrophoretic systems.2–5 Proof of the growing interest in this technique in the scientific community is the exponential growth of ECL papers devoted to analytical chemistry (3056 papers in the last 20 years, doubling every 7 years).The ECL can be carried out mainly by two ECL pathways: hot electron-induced and co-reactant. In hot electron-induced pathways, the ECL emission is initiated by hot energetic electrons from a thin insulator film covered in a conductor/insulator/electrolyte surface during cathodic pulse polarization.6 Sun et al.7 demonstrated that screen-printed carbon electrodes, instead of the common Al/Al2O3 electrode, can generate hot electrons; they proposed procedures for quercetin and dissolved oxygen determination.8
However, more common is the co-reactant ECL pathway where one-directional potential scanning is applied on the electrode in the presence of a luminophore and co-reactant, generating co-reactant intermediates that are either strong reducing agents (in oxidative-reduction ECL) or strong oxidizing agents (in reductive-oxidation ECL).9
When this co-reactant approach is used for disposable format sensors, immobilization is needed for the sensing chemistry, including luminophores; different immobilization strategies are used depending on the analytical scheme utilized and the type of luminophore.
In the case of ruthenium complexes as luminophores, mainly Ru(II)-bis(2,2′-bipyridyl), the common procedures for immobilization consist of a cationic exchange polymer, such as Nafion;10 inclusion in a self-assembled monolayer of ruthenium complex-aminopropyl imidazole on a gold-deposited screen printed cell containing carbon nanotubes;11 or adsorption on electrochemically oxidized graphite in screen printed cells.12
However, the most popular chemistry is based on luminol, for which different forms of immobilization have been proposed including entrapment in membranes, ion-exchange, covalent bonding and electropolymerization.13 The ability of luminol to undergo electropolymerization by cyclic voltammetry is an interesting option to produce electrochemiluminescent polymers on the transducer surface. These have been prepared on graphite, glassy carbon, platinum, gold, screen-printed and indium tin oxide electrodes, typically in acidic conditions.14–20
In previous papers, we studied the improvement in luminol immobilization as a copolymer with 3,3′,5,5′-tetramethylbenzidine13 or biotinylated pyrroles21 for the preparation of disposable ECL sensors by means of electrochemical copolymerization in terms of operational and temporal stability because the polymerization of luminol alone has some drawbacks such as undesirable mechanical properties, luminol adsorption in the polymer, short reusability and poor electroactivity in basic medium.18,22
In order to improve the immobilization of reagents using the electropolymerization process, two approaches can be used: (a) hydrogels and (b) ionogels. In the case of hydrogels, i.e. gels based in water, the hybrid described by Montilla et al.23 can be cited, where silica gel is used to produce polyaniline nanowires by electrodeposition, generating a polyaniline/silica composite material. With the aim of improving the ability of an ECL sensing film to host biorecognition elements and better hydration conditions, recently Loïc Blum et al.24 have developed electrochemiluminescent biosensors based on hybrids of electrogenerated polyluminol nanowires and hydrogels such as silica and calcium alginate for hydrogen peroxide and choline detection.
The second approach for reagent immobilization consists of the preparation of ionogels, a new class of hybrid materials, characterized by the incorporation of ionic liquids (ILs) in the gel. ILs are salts of organic cations such as ammonium, phosphonium, imidazolium, or pyridinium.25 These salts are characterized by their high ionic conductivity and their wide electrochemical potential window (up to 6.0 V). At this time they are widely considered as “green” materials due to their possible recyclability in most applications26 and for their chemical stability. One of the reasons that ionogels can be suitable for ECL analysis is the improvement in both electrochemical and luminescent properties by the ready diffusion of analytes into the immobilized IL phase.25
To date, ionogels consist of ILs hybridized with other components to improve the membrane properties, which may be the following: (1) organic, (2) inorganic or (3) hybrid organic–inorganic (e.g. polymer and inorganic fillers).27
The first case includes two modalities: (a) organic ionogels where low molecular weight gelators are used such as L-glutamic or aspartame acids and (b) polymer gels, which combine the mechanical flexibility of a polymer and the characteristic conductivity, such as the described compatibility of imidazolium salts with poly(methylmethacrylate), among others.
The second case is based on inorganic ionogels called “bucky” gels, such as carbon nanotubes dispersed in ILs where a gelation effect occurs (crosslinking effect), and silica-based ionogels. Silica is a suitable matrix because its properties, such as pore size, orientation, and shape, can be easily tuned during synthesis.28 In this case, two modalities can be distinguished: (a) dispersion of silica nanoparticles into ILs, where bare silica colloids were shown to be unstable in ILs, and (b) sol–gel processing, where the IL can be used as a template that is removed at the end of the gelation or as an enhancer of the conductivity, e.g. monolithic materials.
With respect to the possibilities of ECL sensor preparation using polymers, we focused on the third case, composite (hybrid organic–inorganic) ionogels that consist of the association of molecular organogelators (polymers) and inorganic nanoparticles providing flexibility, thermal stability, and ionic transport. As an example, Leroux et al.29 prepared polymer nanocomposites by incorporating silica nanofillers of silicon tetraethyloxide covalently bonded to the polymer chains as poly(methylmethacrylate) with imidazolium salts as bis-(trifluoromethanesulfonyl)imide.
Due to the potential of this last type of ionogel, this paper explores the feasibility of preparing ionogels using different ILs on screen printed cells for sensing purposes, combining organic materials such as the copolymer luminol with 3,3′,5,5′-tetramethylbenzidine as luminophore and chitosan with inorganic silica by means of electrochemical growth of copolymer nanowires in the pores of the ionogel matrix. To date, this configuration has not been used and is proposed in this work.
2. Experimental
2.1 Chemicals
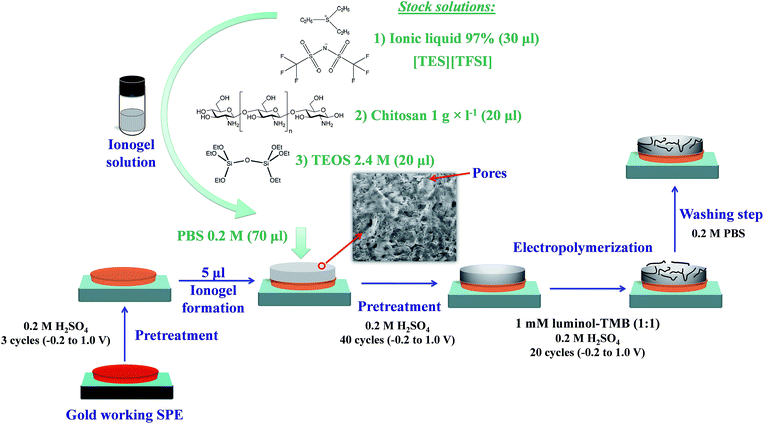
As monomers for electropolymerization, stock solutions of 1 mM of luminol (5-amino-2,3-dihydro-1,4-phthalazinedione), 97% and 3,3′,5,5′-tetramethylbenzidine, 97% (TMB), both supplied by Sigma (Sigma-Aldrich Química S.A., Madrid, Spain, https://www.sigmaaldrich.com/spain.html) were used. A stock buffer phosphate solution containing 0.5 M phosphate (Na2HPO4) and 0.25 M NaCl adjusted to the necessary pH by adding NaOH or HCl of appropriate concentrations was prepared. Different ionic liquids (ILs) were used, such as triethylsulfonium bis(trifluoromethylsulfonyl)imide ([TES][TFSI], 97%), 1-butyl-3-methylimidazolium tetrafluoroborate ([BMIM][BF4], 97%), 1-ethyl-3-methylimidazolium tetrafluoroborate ([EMIM][BF4], 97%), 1-decyl-3-methyl-imidazolium chloride ([decyl-MIM][Cl], 96%), 1-hexyl-3-methyl-imidazolium hexafluorophosphate ([HMIM][PF6], 97%), 1-dodecyl-3-methyl-imidazolium iodide ([dodecyl-MIM][I], 95%); 1-hexyl-3-methyl-imidazolium tetrafluoroborate ([HMIM][BF4], 97%), 1-methyl-3-octyl-imidazolium chloride ([MOIM][Cl], 97%), and 1-butyl-3-methyl-imidazolium hexafluorophosphate ([BMIM][PF6], 97%). Hydrogen peroxide solutions were prepared daily from H2O2 33% w/v (110 vol) that was supplied from Panreac (Panreac Química S.L, Barcelona, Spain, http://www.panreac.es/) and was standardized iodimetrically. Other reagents and materials used were as follows: 1,1′-ferrocenedicarboxylic acid (97%) and Nafion 5% w/w solution in a mixture of aliphatic alcohols (Sigma-Aldrich). Chitosan was prepared by dissolving 1 g in 100 ml water containing 1 ml of acetic acid (95.5%, Panreac) and stirring for 4 hours; the prepared dilution of 1 g l−1 stock solution of chitosan was prepared by dilution with 1% acetic acid. Tetraethyl orthosilicate (TEOS) stock solution (2.4 M) was prepared by mixing the following under vigorous stirring at room temperature, 4.46 ml of TEOS, reagent grade 98% (Sigma-Aldrich), 1.44 ml of water and 6 μl of 4.0 M HCl in a closed vial for 2 h. After that, 1 ml of this solution was mixed carefully with 1 ml of water and submitted to rotaevaporation with periodic control of the weight loss to 0.62 g due to the elimination of ethanol by an alkoxide hydrolysis reaction. The composite was prepared using the stock solutions indicated above (see Fig. 1), obtaining the ionogel solution. Reverse-osmosis type quality water (Milli-Q Plus185 from Millipore, Molsheim, France) was used throughout.2.2 Apparatus and software
The ECL emission from screen-printed cells was measured using an H8529 photomultiplier (PMT) interfaced to a C8855 USB photo counting unit, both from Hamamatsu (Hamamatsu Technologies K.K., Shizuoka, Japan), connected to a PC. The potentiostat used was an Autolab PGSTAT 128N with an F120 module for chrono-coulometric measurements (Metrohm Autolab B.V., Utrecht, Netherlands). The arrangement used for ECL studies has been described in previous works30 and basically consists of a black box that contains two black methacrylate piece holders, where one piece fits on top of the other, one to insert the electrochemical cell in a fixed position and the other to hold the PMT.An electrochemical quartz crystal microbalance (EQCM) (Metrohm Autolab B.V.) was used to study the polymerization process by recording the change in resonant frequency of a 6 MHz EQCM Crystal Au/TiO2 quartz crystal oscillator. A Cary Eclipse fluorescence spectrometer (Varian Australia Pty Ltd.) was used for luminescence measurements. As the conventional Scanning Electron Microscopy technique induces an instantaneous dehydration of the sample due to the high vacuum needed, we used environmental scanning electron microscopy (ESEM), which makes it possible to examine any specimen, wet or dry, insulating or conducting, in situ and close to its natural state. An ESEM FEI model Quanta (FEI Co., Hillsboro, Oregon, USA) 3.5 nm was used for imaging with increases ranging from 7× to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000× and equipped with a ZEISS DSM 250 electronic microscope prepared to perform energy dispersive X-ray spectroscopy (EDX) microanalysis. Samples were mounted on Peltier cooling stages set at 2 °C with a working distance of about 10 mm and a humidity of 80%. A Crison digital pH-meter (Crison Instruments, Barcelona, Spain) with a combined glass-saturated calomel electrode was used for pH measurement.
000× and equipped with a ZEISS DSM 250 electronic microscope prepared to perform energy dispersive X-ray spectroscopy (EDX) microanalysis. Samples were mounted on Peltier cooling stages set at 2 °C with a working distance of about 10 mm and a humidity of 80%. A Crison digital pH-meter (Crison Instruments, Barcelona, Spain) with a combined glass-saturated calomel electrode was used for pH measurement.
Software programs used were: Statgraphics Centurion software package (Manugistics Inc. and Statistical Graphics Corporation, USA, 2007); Microsoft Office Suite 2013 (Microsoft Corp., Redmond, WA, USA); CSW32 v.1.3.3 (2001); CSWAIA v 1.7.3 (2001) (Dataapex software, Czech Republic); NOVA v.1.8.17 (2005–2012) (Metrohm Autolab B.V.) and GPES v.4.9 (2007) (Ecochemie, Netherlands).
2.3 SPE cells and pre-treatments
Low temperature (LT) gold screen-printed cells (SPE) were selected for sensor preparation based on our previous studies of a convenient electropolymerization of aniline derivatives.13 These cells were supplied by Dropsens S.A. (Oviedo, Spain, http://www.dropsens.com/). The cells consist of a round-shaped working electrode and a counter electrode, both of the same material, and a silver pseudo-reference electrode on a ceramic support. Before being used, the electrochemical cells were tested for uniform behaviour. In order to prepare a receptacle on the disposable electrochemical cell, we printed the round form on the working electrode by screen printing using a plastisol ink containing 30% Puff additive, which expands during heating (170–200 °C), giving a raised print used as a receptacle of approximately 50 μl capacity.Initial pre-treatment of gold SPE cells was carried out by immersing them in 0.2 M H2SO4 and applying three voltammetric sweeps between −0.2 and 1.0 V. Then, the SPE cells were dried for 10 min at room temperature. After the addition of the ionogel solution to the SPE cell (as shown in the following section), a second pre-treatment was carried out by immersing the cell in the same 0.2 M H2SO4 solution and applying 40 voltammetric sweeps between −0.2 and 1.0 V. For the two pre-treatments, 0.1 V s−1 was set as the scan speed.
2.4 Ionogel sensor preparation
An ionogel solution was prepared by mixing 20 μl TEOS stock solution (2.4 M), 20 μl of 1.0 g l−1 chitosan solution and 30 μl of [TES][TFSI] stock solution in an ultrasonic bath. This ionogel solution can be used for a week if maintained at −20 °C. To prepare the composite and prompt the gelation, 70 μl of pH 7.4 PBS solution containing 0.2 M phosphate and 0.25 M NaCl was added finally to the inonogel solution. 5 μl of this mixture was dropped onto the working electrode of the gold LT SPE cell, waiting for two minutes at room temperature after use.After the second pre-treatment, the luminol–TMB copolymer was grown in the pores of the prepared composite on the SPE cell by cyclic voltammetry working between −0.2 and 1.0 V, at 0.1 V s−1, with 20 cycles from a solution of 1 mM luminol and TMB (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio) in 0.2 M H2SO4 (standard conditions). Then, the sensor was washed with 0.2 M pH 7.4 phosphate buffer solution to eliminate adsorbed luminol or TMB traces. The prepared cells were stored in dark conditions at 4 °C until use (see Fig. 1).
1 ratio) in 0.2 M H2SO4 (standard conditions). Then, the sensor was washed with 0.2 M pH 7.4 phosphate buffer solution to eliminate adsorbed luminol or TMB traces. The prepared cells were stored in dark conditions at 4 °C until use (see Fig. 1).
2.5 ECL procedure and signal measurements
The experimental system for ECL measurement has been described elsewhere using the instrumentation shown in the apparatus and software section.30 Aqueous standard solutions of different concentrations of H2O2 at pH 9.0 adjusted with a stock solution of saline phosphate buffer were prepared. 50 μl of the H2O2 solution was deposited into the receptacle of the SPE, which was then placed in a homemade holder and covered with a lid holding the PMT. The ECL signal obtained for analytical purposes was the integral signal over the course of 2 min coming from consecutives pulses of 0.6 V for 1 s each with a dwell time of 10 s.3. Results and discussion
The goal of this study is the preparation and characterization of SPE cells with an ionogel composite31 with biocompatibility and biodegradability properties for electrochemiluminescent detection. This biocomposite is prepared by a ionogel solution that consists of the biopolymer chitosan, TEOS stock solution and an IL, inserting ECL nanowires into the pores of the solid ionogel prompted by 0.2 M PBS solution by in situ copolymerization of luminol and an aromatic amine: 3,3′,5,5′-tetramethylbenzidine. A study of biocomposite electro-preparation and ECL characterization on different SPE cells was performed.3.1 SPE sensor composition
In the first instance, a sol–gel configuration was used by immobilizing the electroformed poly(luminol–TMB) as nanowires, obtaining an intense ECL signal with H2O2 (see Table 1). However, the membrane was brittle and cracked some minutes after preparation due to the rapid dehydration of the membrane, resulting in an unstable sensor. To overcome the observed deficiencies it was necessary to include other components in the membrane in order to produce a hydrogel. Nafion, an anionic sulfonated perfluorinated vinyl ether polymer, widely used as an ion-exchange polymer, is described as a hydration agent in the preparation of sol–gel sensors by water adsorption at the hydrophilic ionic groups forming ionic clusters.34 In this case, its use for composite preparation generated a reduction in the ECL signal of approximately 60% compared to the cell containing only sol–gel, and a retraction in the membrane with fast cracking in a few minutes. This behaviour can be attributed to the fact that nafion decelerates the electron transfer as well as the diffusion of luminol.35
| Composite | Layer composition | ECL analytical signal a.u. s | % |
|---|---|---|---|
| Sol–gel | TEOS | 397 | 84.2 |
| Hydrogel | TEOS/nafion | 77 | 16.3 |
| TEOS/chitosan | 311 | 66.1 | |
| TEOS/chitosan/nafion | 155 | 32.8 | |
| TEOS/chitosan/nafion/ferrocene | 11 | 2.3 | |
| TEOS/chitosan/ferrocene | 20 | 4.2 | |
| Ionogel | TEOS/chitosan/[TES][TFSI] | 472 | 100.0 |
Moreover, chitosan, a positively charged natural polysaccharide, generates a sol–gel that is highly porous and structured, creating a hydrophilic environment.36 The biocomposite is prepared by entrapping the chitosan in the porous silica matrix when the pH of the solution containing sol nanoparticles of silica and chitosan is neutralized, enabling crosslinking. In this biocomposite, the electron mobility is reduced as is, consequently, the ECL signal to some extent by approximately 21.5% with respect to the SPE cell containing only sol–gel, although the biocomposite membrane shows good mechanical properties.
The combined use of chitosan and nafion along with sol–gel in a hydrogel did not provide any differential characteristics, only an increase in the ECL signal with respect to that of nafion (50.4%) and a decrease with respect to that of chitosan (50.3%). With the goal of improving the ECL signal in the chitosan/sol–gel material, we studied the effect of the incorporation of electronic mediators and ionic conductors. The inclusion of a typical electronic mediator such as 1,1′-ferrocene dicarboxylic acid, generally used as an electronic transporter in sensing membranes,37 dramatically reduced the ECL emission, centred at 440 nm, approximately 95% compared to the sol–gel layer, due to the absorption of ferrocene at 450 nm.
Contrastingly, the incorporation of ionic liquids (ILs) produces an ionogel that shows a large increase in the ECL signal, which makes these liquids useful for biocomposite preparation. ILs can be classified, according to their preparation, in three generations:38 (1) those based on 1-alkyl-3-methylimidazolium salts with tetrachloroaluminates or halides (based on eutectics); (2) those obtained by replacing the anion by tetrafluoroborate or other anions (discrete anions); (3) covalent inclusion of a functional group in the cation, anion or both. In this study, we tested different ionogels with silica and chitosan prepared from ILs belonging to three generations (see Table 2). The results show that ILs from the first generation prevent the preparation of the biocomposite due to solubility problems, an aspect that is improved in second generation ILs due to their hydrophilic properties, which produce a water-stable composite. However, the produced ECL intensity reached only 21% of the maximum. The use of the third generation IL triethylsulfonium bis(trifluoromethylsulfonyl)imide ([TES][TFSI]) dramatically increased the ECL intensity.
| Composite | Generation | IL | ECL analytical signal a.u. s | % |
|---|---|---|---|---|
| Ionogel (TEOS/chitosan/IL) | First | [Decyl-MIM][Cl] | Solubility problems | — |
| [Dodecyl-MIM][I] | ||||
| [MOIM][Cl] | ||||
| Second | [HMIM][PF6] | — | — | |
| [EMIM][BF4] | 74 | 15.7 | ||
| [BMIM][BF4] | 87 | 18.5 | ||
| [HMIM][BF4] | — | — | ||
| [BMIM][ PF6] | 98 | 20.9 | ||
| Third | [TES][TFSI] | 472 | 100.0 |
The different behaviour of ionogels containing the studied ILs can be justified by their fluorescence properties. The ILs containing bulky imidazolium cations showed absorption bands around 350 nm with a fluorescence emission near 480 nm, although their confinement in the silica matrix modifies the ground and excited states involved in fluorescence, shifting their absorption and emission maxima, i.e., the emission peak, from 484 to 579 nm in the case of [BMIM][PF6], consequently reducing the ECL emission due to absorption by imidazolium ILs.39 In turn, the [TES][TFSI] showed an absorption band at 373 nm with the emission band at 431 nm, which apparently did not affect the ECL process. Thus, the [TES][TFSI] containing triflimide anion was selected. This IL is widely used for its relatively low viscosity and high ionic mobility and thus, for the electro-formation of conducting polymers,40 such as polypyrrole41 and poly(3-methylthiophene).42
A cyclic voltammogram on a gold SPE cell using 0.2 M H2SO4 solution (Fig. 2A(a)) showed an oxidation peak at 1.0 V corresponding to the oxidation of gold by water, and subsequently a reduction peak at 0.75 V due to the reduction of Au oxides formed in the anodic reaction on the working electrode. The placement of a sol–gel layer on the working electrode (Fig. 2A(b)) dramatically reduced the oxidation and reduction peaks due to the low conductivity of the material. The inclusion of chitosan in the sol–gel layer does not apparently improve the electrochemical properties (Fig. 2A(c)). The contribution of ionic liquid to the conductivity is highlighted in Fig. 2A(d), showing left-shifted gold oxidation and reduction peaks that result in a modified SPE electrode that improves the electrochemical characteristics of the bare gold SPE electrode.
To study the electro-formation of the copolymer, first a bare Au SPE cell was used with luminol and TMB solutions working under the same electropolymerization conditions as previously studied by our group13 (between −0.2 and 1.0 V, at 0.1 V s−1, from 1 mM solution of both luminol and TMB (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio) in 0.2 M H2SO4), obtaining the results in Fig. 2B(a).
1 ratio) in 0.2 M H2SO4), obtaining the results in Fig. 2B(a).
A study of luminol–TMB copolymer formation by cyclic voltammetry in the ionogel TEOS/chitosan/IL biocomposite is shown in Fig. 2C.
An increase in the intensity and left-shifted anodic and cathodic peaks was observed for copolymerization on a sol–gel layer on an Au SPE cell (Fig. 2B(b)). However, this membrane experienced a quick cracking effect due to poor hydration. The polymerization in the hydrogel TEOS/chitosan biocomposite produced a dramatic decrease in the intensity of the voltammetric peaks that appeared at the same voltage (Fig. 2B(c)). Finally, the inclusion of [TES][TFSI] IL in the biocomposite improved the formation of the copolymer (Fig. 2B(d)).
A study of luminol–TMB copolymer formation by cyclic voltammetry in the ionogel TEOS/chitosan/IL biocomposite is shown in Fig. 2C. The same previously described pattern can be observed:33 TMB oxidation peak at 0.60 V; luminol oxidation peak at 0.89 V; copolymer oxidation at 0.63 V and reduction at 0.54 V. The EQCM study corroborates the electro-formation of the copolymer (Fig. 2D), showing a progressive increase in the mass by decreasing the resonance frequency by cycling. Using the Sauerbrey equation,43 the calculated mass of the deposited copolymer after 20 cycles was 674.8 ng cm−2.
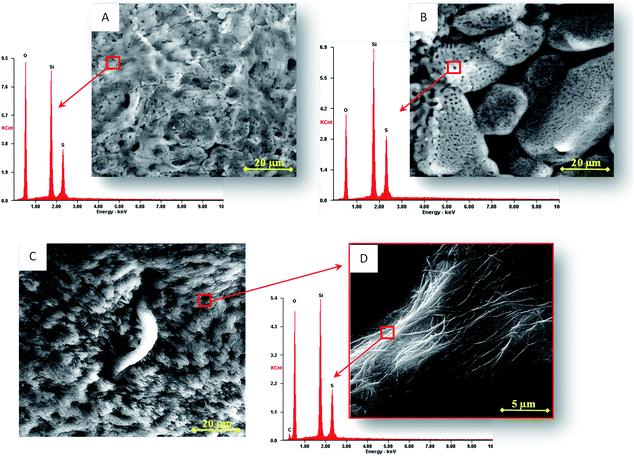
The superficial topology of the porous biocomposite (Fig. 3B), obtained after the second pre-treatment, prepares the composite for the growth of copolymer strands in the porous structure (5 μm of pore size), giving rise to a massive growth of poly(luminol–TMB) nanowires (Fig. 3C). An interconnected filament shaped structure is observed when enlarging the image (Fig. 3D) in which the EDX spectrum confirms the presence of carbon from the copolymer and sulphur that can arise from either hydrogen sulfate or triflimide anions. In the latter case, this is the product of the ionic liquid that has been described as a dopant in polypyrroles.41
Once the electrode with the biocomposite was prepared and before the electropolymerization process, a new pre-treatment step was necessary to improve the immobilization of the copolymer and the stability of the ionogel membrane. The morphology of the composite (Fig. 3A) is considerably modified after pre-treatment, increasing the number and distribution of the pores (Fig. 3B) and improving the nanowire formation on them.
After 40 cycles in the same previous conditions, a better electro-formation of the copolymer occurred after the second pre-treatment. This second pre-treatment can have three functions: (a) a polishing effect that eliminates traces or extra components of the composite; (b) a removing effect that permits the pore walls to strengthen on aging because some removed IL acts as a template,27 as we can observe by an EQCM study where a constant loss of mass occurred after each new pre-treatment cycle, resulting in a mass loss of 858.9 ng cm−2 (after 40 cycles); and (c) an interchange of the counter ion acetate present in chitosan by hydrogen sulphate. This is sustained by the EDX study that shows the presence of sulphur that was not present earlier in the composite structure after pre-treatment (see Fig. 3A and B). The increase in hydrogen sulphate in the membrane favours polymerization due to the fact that it acts as a dopant agent in copolymer formation.44
The influence of the [TES][TFSI] concentration on the composite was studied between 0.2 and 1.2 M with 0.14 g l−1 chitosan. The ECL values show low variation between 0.2 and 0.7 M in IL, decreasing afterwards due to the same reason discussed above: an increase in the autoabsorption due to an increase in the amount of copolymer formed and thus in the colour intensity. The final composition selected for the biocomposite was 0.14 g l−1 chitosan, 0.7 M [TES][TFSI] and 0.35 M of TEOS.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 monomer ratio) in the optimized conditions discussed above, the analytical signal grew up to 20 cycles, decreasing afterwards (see Fig. 4B, red line). This is attributed to an increase in the mass of the formed nanowires along the number of cycles, which generates a self-absorption of the ECL emission (emission wavelength at 450 nm) produced by the increased formation of diimine TMB2+ cations (absorption wavelength at 450 nm) on the electrode surface.46 Fig. 4B shows that in the absence of IL, the ECL signal decreased, probably due to a less efficient electro-formation process. An increase in the cycle number is necessary in cells prepared without IL to achieve similar signals (up to 60 cycles).
1 monomer ratio) in the optimized conditions discussed above, the analytical signal grew up to 20 cycles, decreasing afterwards (see Fig. 4B, red line). This is attributed to an increase in the mass of the formed nanowires along the number of cycles, which generates a self-absorption of the ECL emission (emission wavelength at 450 nm) produced by the increased formation of diimine TMB2+ cations (absorption wavelength at 450 nm) on the electrode surface.46 Fig. 4B shows that in the absence of IL, the ECL signal decreased, probably due to a less efficient electro-formation process. An increase in the cycle number is necessary in cells prepared without IL to achieve similar signals (up to 60 cycles).3.2 Sensor measurement conditions
As could be expected in a ECL sensor based on a luminol–TMB copolymer, the luminol reactivity against H2O2 increased with the basicity of the medium (pH > 8.0) due to the contribution of hydroxyl groups in catalysing the ECL reaction.47 As in previous works, the blank signal increased with the pH but its standard deviation grew more strongly than the signal at a pH higher than 9.5.13 For this reason, pH 9.0 (phosphate buffer) was selected as the working pH. The volume of sample placed in the receptacle of the SPE cell has an effect on the ECL signal, with the ECL signal increasing with an increase in volume, up to 50 μl (receptacle maximum capacity).To optimize the value of the electrode potential applied, a study using cyclic voltammetry was performed obtaining the maximum ECL emission at 0.6 V, a behaviour similar to that previously observed for poly(luminol–TMB) on bare gold SPE cells,13 suggesting that this new biocomposite maintains its electrochemical properties due to the use of chitosan and IL.
3.3 Mechanism of the ECL reaction
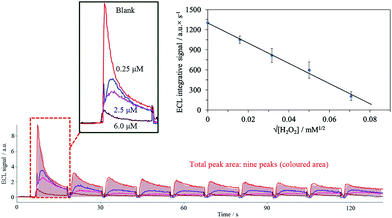
Luminol exhibits a blank ECL signal in the presence of oxygen due to the radical oxidation of luminol with the superoxide anion generated from the dissolved oxygen (eqn (1) and (2)).48,49 The stabilization of the superoxide anion by the ionic liquid [TES][TFSI] due to the strong ionic interactions between cationic IL and anionic superoxide (O2˙−), according to Mjalli et al.50 increases the blank signal (see the expanded image of Fig. 5 and the bar chart of Fig. 4C). The consumption of the dissolved oxygen was observed when several electrochemical cycles were performed, with a decrease in the signal each time (Fig. 5).| O2 + e → O2˙−, E0 = −0.16 V | (1) |
| Luminol + O2˙− → 3APA* → 3APA + hν | (2) |
Moreover, imidazolium containing ionic liquids generate an unstable superoxide because the superoxide anion reacts with the imidazolium cation, giving the corresponding 2-imidazolones,50 which reinforces the reason for the low ECL signal in imidazolium containing ionic liquids in the preliminary studies in Section 3.1.
In the presence of hydrogen peroxide, the usual increase of the ECL signal due to the chemical reaction of hydrogen peroxide and diazaquinone from luminol is observed in typical ECL sensors and less in hydrogel (TEOS/chitosan). This is not evidenced in the ionogel sensor (TEOS/chitosan/[TES][TFSI]) (see bar chart in Fig. 4C) because a decrease in the ECL intensity due to the addition of hydrogen peroxide is observed, which decreases with the concentration. One reason for this could be the conversion of the electroformed superoxide to oxygen with the addition of H2O2 following the Haber–Weiss reaction.51 In general, this reaction requires Fe(III) as a catalyst (Fenton reaction), but it can be electrochemically catalyzed at 0.48 V (pH 7.0), according to Koppenol,51 with the consequent elimination of the superoxide anion from the media (eqn (3)).
| O2˙− + H+ + H2O2 → O2 + HO˙ + H2O, 0.48 V = ΔE0 | (3) |
For that reason, the sensor presented in this paper is sensitive to H2O2, acting as a scavenger of the increased blank signal generated by the biocomposite including the [TES][TFSI] ionic liquid as mediator.
3.4 ECL sensor analytical characterization
The lifetime of the disposable sensor was determined using a series of prepared sensors, regularly testing their response to a 5.0 μM H2O2 concentration. These sensors, when protected from light and kept at 4 °C, can be used for a month without substantial change. At room temperature and in the dark, the sensors can be used for a week, without the signal changing below 10% (254 ± 23 a.u. s). After that, the signal decreased some 20% on each subsequent day. The prepared sensors must be used in disposable format because each sensor cannot be reused more than 10 times, as we confirmed working at 5.0 μM H2O2, after which they showed a decrease in the signal of approximately 35% for each use.
A comparative study between different electrochemiluminescent and amperometric sensors from the bibliography (see Table 4) shows that these methods offer more than three orders of magnitude for H2O2 response, as observed in the sensor presented here (three orders). With respect to the limit of detection, the value found (0.8 nM) is in accordance with the results obtained by other researchers. In the case of the reproducibility, the obtained values from the bibliography using conventional electrodes are better than our results (8.5%), due to the fact that they do not work in disposable format.
| System | Linear range μM | LOD nM | Linearity | Detection | Reproducibility |
|---|---|---|---|---|---|
| Hydrogel:53 tetramethoxysilane/chitosan | 250 to 3400 | 3000 | 0.998 | Amperometry | 4.0% |
| Dendrimer:54 titanate nanotubes/poly(amidoamine) and luminol | 0.001–0.9 | 1.0 | 0.998 | ECL | — |
| Composite:55 titanate nanotubes/nafion and luminol | 0.001–0.5 | 0.88 | 0.998 | ECL | 2.1% |
| Composite:56 poly(luminol–benzidine) | 0.0002–0.1 | 0.060 | 0.997 | ECL | 5.0% |
| Composite:13 poly(luminol–TMB) | 0.0061–0.1 | 2.6 | 0.984 | ECL | 10.2% |
| Hydrogel and nanowires:24 polyluminol/calcium alginate | 0.4–5000 | — | — | ECL | — |
| Ionogel and nanowires: (This work) TEOS/chitosan/IL and poly(luminol–TMB) | 0.0092–7.4 | 0.8 | 0.998 | ECL | 8.5% |
4. Conclusions
The use of a ionogel biocomposite prepared by the solution method from chitosan and ionic liquid in a silica matrix to host nanowires of poly(luminol–TMB) prepared by in situ electropolymerization in the pores of a ionogel make it possible to prepare a disposable reagentless electrochemiluminescent sensor with good mechanical, conductivity and luminescence properties, as well as good operational and temporal stability.The preparation of ionogels with the inclusion of [TES][TFSI] as the ionic liquid containing triflimide anion produces: (a) an interesting increase in the conductivity of the biocomposite layer needed for ECL generation, (b) an increase in hydration along with chitosan needed for silica matrix stabilization, and (c) strong ionic interactions that stabilize the superoxide anion formed from the oxygen present, giving rise to an intense blank ECL emission.
The developed sensor is sensitive to hydrogen peroxide that acts as a scavenger of the stabilized superoxide anion, reducing the ECL signal with the increase in the H2O2 concentration, resulting in a reagentless disposable sensor with a linear range of three orders of magnitude but a better resolution than with usual disposable sensors because the acquisition of the luminescence signal is based on the measurement of the total luminescence area using a photomultiplier detector, such as an enhanced CCD camera. The modified SPE cell studied paves the way for the preparation of biomaterials such as oxidase-based, because binding ionogel with ECL polymers can offer new possibilities for disposable ECL biosensors.
Acknowledgements
We acknowledge financial support from the Junta de Andalucía (Proyecto de Excelencia P10-FQM-5974) and from the Ministerio de Economía y Competitividad (Spain) (CTQ2013-44545-R). These projects were partially supported by European Regional Development Funds (ERDF). We would like to acknowledge Manuel Agudo Acemel for their helpful and kind assistance.Notes and references
- A. Kapturkiewicz, Adv. Electrochem. Sci. Eng., 1997, 5, 1–60 CrossRef CAS.
- K. Muzyka, Biosens. Bioelectron., 2014, 54, 393–407 CrossRef CAS PubMed.
- M. Santhiago, E. W. Nery, G. P. Santos and L. T. Kubota, Bioanalysis, 2014, 6, 89–106 CrossRef CAS PubMed.
- S. Deng and H. Ju, Analyst, 2013, 138, 43–61 RSC.
- L. Hu and G. Xu, Chem. Soc. Rev., 2010, 39, 3275–3304 RSC.
- R. Pyati and M. M. Richter, Annu. Rep. Prog. Chem., Sect. C: Phys. Chem., 2007, 103, 12–78 RSC.
- A. H. Wu, J. j. Sun, Y. M. Fang, R. J. Zheng and G. N. Chen, Electroanalysis, 2010, 22, 2702–2707 CrossRef CAS.
- R. J. Zheng, Y. M. Fang, S. F. Qin, J. Song, A. H. Wu and J. j. Sun, Sens. Actuators, B, 2011, 157, 488–493 CrossRef CAS PubMed.
- W. Miao, Chem. Rev., 2008, 108, 2506–2553 CrossRef CAS PubMed.
- Y. Xu, B. Lou, Z. Lv, Z. Zhou, L. Zhang and E. Wang, Anal. Chim. Acta, 2013, 763, 20–27 CrossRef CAS PubMed.
- C. H. Kang, Y. B. Choi, H. H. Kim, H. N. Choi and W. Y. Lee, Electroanalysis, 2011, 23, 2131–2138 CrossRef CAS.
- J. Ballesta-Claver, R. Rodriguez-Gomez and L. F. Capitán-Vallvey, Anal. Chim. Acta, 2013, 770, 153–160 CrossRef CAS PubMed.
- J. Ballesta-Claver, M. C. Valencia-Mirón and L. F. Capitán-Vallvey, Anal. Bioanal. Chem., 2011, 400, 3041–3051 CrossRef CAS PubMed.
- Y. T. Chang, K. C. Lin and S. M. Chen, Electrochim. Acta, 2005, 51, 450–461 CrossRef CAS PubMed.
- S. M. Chen and K. C. Lin, J. Electroanal. Chem., 2002, 523, 93–105 CrossRef CAS.
- C. H. Wang, S. M. Chen and C. M. Wang, Analyst, 2002, 127, 1507–1511 RSC.
- G. F. Zhang and H. Y. Chen, Anal. Chim. Acta, 2000, 419, 25–31 CrossRef CAS.
- A. Sassolas, L. J. Blum and B. D. Leca-Bouvier, Anal. Bioanal. Chem., 2008, 390, 865–871 CrossRef CAS PubMed.
- A. Sassolas, L. J. Blum and B. D. Leca-Bouvier, Anal. Bioanal. Chem., 2009, 394, 971–980 CrossRef CAS PubMed.
- A. Sassolas, L. J. Blum and B. D. Leca-Bouvier, Sens. Actuators, B, 2009, 139, 214–221 CrossRef CAS PubMed.
- J. Ballesta-Claver, J. Ametis-Cabello, J. Morales-Sanfrutos, A. Megia-Fernandez, M. C. Valencia-Mirón, F. Santoyo-Gonzalez and L. F. Capitán-Vallvey, Anal. Chim. Acta, 2012, 754, 91–98 CrossRef CAS PubMed.
- G. Li, J. Lian, X. Zheng and J. Cao, Biosens. Bioelectron., 2010, 26, 643–648 CrossRef CAS PubMed.
- F. Montilla, M. A. Cotarelo and E. Morallon, J. Mater. Chem., 2009, 19, 305–310 RSC.
- B. Leca-Bouvier, A. Sassolas and L. J. Blum, Anal. Bioanal. Chem., 2014, 1–11 Search PubMed.
- A. Vioux, L. Viau, S. Volland and J. Le Bideau, C. R. Chim., 2010, 13, 242–255 CrossRef CAS PubMed.
- A. I. Horowitz, Y. Wang and M. J. Panzer, Green Chem., 2013, 15, 3414–3420 RSC.
- J. Le Bideau, L. Viau and A. Vioux, Chem. Soc. Rev., 2011, 40, 907–925 RSC.
- R. Gobel, P. Hesemann, J. Weber, E. Moller, A. Friedrich, S. Beuermann and A. Taubert, Phys. Chem. Chem. Phys., 2009, 11, 3653–3662 RSC.
- F. Gayet, L. Viau, F. Leroux, F. Mabille, S. Monge, J. J. Robin and A. Vioux, Chem. Mater., 2009, 21, 5575–5577 CrossRef CAS.
- J. Ballesta-Claver, M. C. Valencia-Miron and L. F. Capitán-Vallvey, Analyst, 2009, 134, 1423–1432 RSC.
- Y. Shchipunov, Pure Appl. Chem., 2012, 84, 2579–2607 CrossRef CAS.
- J. Ballesta-Claver, V. Salinas, M. C. Valencia-Miron and L. F. Capitan-Vallvey, Talanta, 2011, 86, 78–185 CrossRef CAS PubMed.
- J. Ballesta-Claver, I. F. Díaz Ortega, M. C. Valencia-Mirón and L. F. Capitán-Vallvey, Anal. Chim. Acta, 2011, 702, 254–261 CrossRef CAS PubMed.
- C. D. Feng, S. L. Sun, H. Wang, C. U. Segre and J. R. Stetter, Sens. Actuators, B, 1997, 40, 217–222 CrossRef CAS.
- G. Xu, X. Zeng, S. Lu, H. Dai, L. Gong, Y. Lin, Q. Wang, Y. Tong and G. Chen, Luminescence, 2013, 28, 456–460 CrossRef CAS.
- M. A. Kim and W. Y. Lee, Anal. Chim. Acta, 2003, 479, 143–150 CrossRef CAS.
- L. Zhang, X. Gao, L. Yang, P. Yu and L. Mao, ACS Appl. Mater. Interfaces, 2013, 5, 8120–8124 CAS.
- M. Hasanzadeh, N. Shadjou, M. Eskandani and M. d. l. Guardia, TrAC, Trends Anal. Chem., 2012, 41, 58–74 CrossRef CAS PubMed.
- M. P. Singh, R. K. Singh and S. Chandra, ChemPhysChem, 2010, 11, 2036–2043 CAS.
- L. Viau, M. A. Neouze, C. Biolley, S. Volland, D. Brevet, P. Gaveau, P. Dieudonne, A. Galarneau and A. Vioux, Chem. Mater., 2012, 24, 3128–3134 CrossRef CAS.
- I. Villareal, E. Morales, T. F. Otero and J. L. Acosta, Synth. Met., 2000, 108, 57–65 CrossRef CAS.
- C. Fuvre, L. Abello and D. Delubouglise, Adv. Mater., 1997, 9, 722–725 CrossRef.
- V. M. Mecea, Anal. Lett., 2005, 38, 753–767 CrossRef CAS PubMed.
- S. Cosnier and A. A. Karyakin in Electropolymerization: Concepts, Materials and Applications, Wiley-VCH, 1st edn, 2010 Search PubMed.
- A. E. Kadib and M. Bousmina, Chem.–Eur. J., 2012, 18, 8264–8277 CrossRef CAS PubMed.
- P. D. Josephy, T. Eling and R. P. Mason, J. Biol. Chem., 1982, 257, 3669–3675 CAS.
- H. Chu, W. Guo, J. Di, Y. Wu and Y. Tu, Electroanalysis, 2009, 21, 1630–1635 CrossRef CAS.
- W. Wang, H. Cui, Z. X. Deng, Y. P. Dong and J. Z. Guo, J. Electroanal. Chem., 2008, 612, 277–287 CrossRef CAS PubMed.
- O. V. Reshetnyak, E. P. Koval'chuk and J. Blazejowski, Russ. J. Electrochem., 2011, 47, 1111–1118 CrossRef CAS.
- M. Hayyan, F. S. Mjalli, M. A. Hashim, I. M. AlNashef and X. M. Tan, J. Electroanal. Chem., 2011, 657, 150–157 CrossRef CAS PubMed.
- W. H. Koppenol, Redox Rep., 2001, 6, 229–234 CrossRef CAS PubMed.
- J. Mocak, A. M. Bond, S. Mitchell and G. Scollary, Pure Appl. Chem., 1997, 69, 297–328 CrossRef CAS.
- Y. Miao and S. N. Tan, Anal. Chim. Acta, 2001, 437, 87–93 CrossRef CAS.
- Y. Lin, H. Dai, G. Xu, T. Yang, C. Yang, Y. Tong, Y. Yang and G. Chen, Microchim. Acta, 2013, 180, 563–572 CrossRef CAS.
- G. Xu, X. Zeng, S. Lu, H. Dai, L. Gong, Y. Lin, Q. Wang, Y. Tong and G. Chen, Luminescence, 2013, 28, 456–460 CrossRef CAS.
- G. Li, X. Zheng and L. Song, Electroanalysis, 2009, 21, 845–852 CAS.
| This journal is © The Royal Society of Chemistry 2014 |