Synthesis and magnetic properties of MNb2O6 (M = Fe, Co, Ni) nanoparticles
Abstract
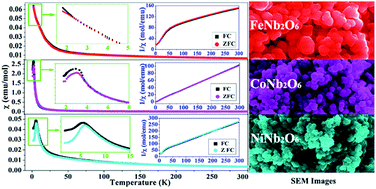
Considerable efforts have been exerted on the controllable synthesis of columbite niobate ceramics due to their fascinating properties and applications. Especially, it is still a great challenge to fabricate nanostructures of the niobate series. In this research, FeNb2O6, CoNb2O6 and NiNb2O6 nanoparticles have been successfully prepared via a facile hydrothermal route followed by heat treatment. X-ray powder diffraction patterns show that all the products have the typical orthorhombic columbite structure. The electron microscopy analyses reveal that the obtained nanoparticles have diameters of 50–100 nm. The magnetic property results demonstrate that the magnetically ordered state is hard to observe down to 1.8 K for the FeNb2O6 sample, while the magnetic transition temperatures of TN = 3 K and TN = 6 K can be obtained for CoNb2O6 and NiNb2O6 samples, respectively. A weak ferromagnetic moment can be detected below 5 K for both CoNb2O6 and NiNb2O6 samples. Furthermore, the NiNb2O6 sample even exhibits a metamagnetic transition at 1.8 K due to the spin flipping of the ferromagnetic chains.

 Please wait while we load your content...
Please wait while we load your content...