“Pulling” π-conjugated polyene biomolecules into water: enhancement of light-thermal stability and bioactivity by a facile graphene oxide-based phase-transfer approach†
Abstract
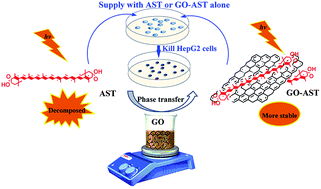
This work demonstrated that graphene oxide (GO) could not only be exploited as a nanovector to efficiently transfer π-conjugated polyene biomolecules from the organic phase to the aqueous phase, but also could enhance light-thermal stability and bioactivity of the transferred π-conjugated polyene biomolecules.

 Please wait while we load your content...
Please wait while we load your content...