Graphene-wrapped sulfur-based composite cathodes: ball-milling synthesis and high discharge capacity
Abstract
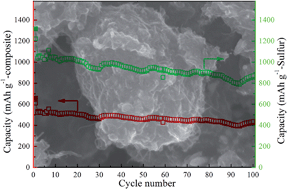
A facile ball-milling route, which can help in large-scale synthesis, is developed to prepare graphene-wrapped sulfur/carbon nanotubes composite. Electrochemical tests show that the obtained composite demonstrates high sulfur utilization and good cycle performance. As calculated by the total mass of the composite, a high reversible capacity of 626.0 mA h g−1 can be obtained after 70 cycles at a current density of 400 mA g−1.

 Please wait while we load your content...
Please wait while we load your content...