Synthesis of a novel CuI/CuII-containing sandwich-type cluster and its catalytic electron transfer property†
Abstract
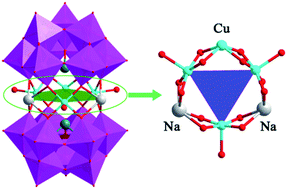
A novel six-metal sandwich-type heteropolyanion [Na2CuICuII(OH2)Cu2II(B-α-SbW9O33)2]9− (1) has been synthesized in good yield from a one-pot hydrothermal reaction and characterized by IR, XPS, XRD and TG. Single-crystal X-ray analysis reveals that compound H9[Na2CuICuII(OH2)Cu2II(B-α-SbW9O33)2]·9H2O (H9-1) crystallizes in tetragonal system with space group of P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 21m and consists of a 2D inorganic network based on Cu–O–W linkages among polyanionic clusters. A structural feature is that the six-metal {Na2Cu4} central belt of the anion contains the mixed valence states of CuI/CuII group. This compound shows excellent catalytic activity for effectively promoting the inorganic electron transfer (redox) reaction of ferricyanide to ferrocyanide by thiosulphate with high rate constant value in aqueous solution. In addition, compound (H9-1) also exhibits an inhibition effect for the organic photodegradation reaction of Rhodamine B (RhB).
21m and consists of a 2D inorganic network based on Cu–O–W linkages among polyanionic clusters. A structural feature is that the six-metal {Na2Cu4} central belt of the anion contains the mixed valence states of CuI/CuII group. This compound shows excellent catalytic activity for effectively promoting the inorganic electron transfer (redox) reaction of ferricyanide to ferrocyanide by thiosulphate with high rate constant value in aqueous solution. In addition, compound (H9-1) also exhibits an inhibition effect for the organic photodegradation reaction of Rhodamine B (RhB).

 Please wait while we load your content...
Please wait while we load your content...