N-arylation of amines: C–N coupling of amines with arylboronic acids using Fe3O4 magnetic nanoparticles-supported EDTA–Cu(II) complex in water†
Ramin Mostafalua,
Babak Kaboudin*a,
Foad Kazemia and
Tsutomu Yokomatsub
aDepartment of Chemistry, Institute for Advanced Studies in Basic Sciences, Gava Zang, Zanjan, Iran. E-mail: kaboudin@iasbs.ac.ir; Fax: +98 241 4214949; Tel: +98 241 4153220
bSchool of Pharmacy, Tokyo University of Pharmacy and Life Sciences, 14321-1 Horinouchi, Hachioji, Tokyo 192-0392, Japan
First published on 16th September 2014
Abstract
Fe3O4 magnetic nanoparticles-supported EDTA–copper(II) complex (Fe3O4-EDTA–Cu(II)) has been prepared and characterized by TEM, SEM, XRD, VSM, TGA, and FT-IR spectrometers. The catalyst was applied for the C–N coupling of arylboronic acids with amines for the preparation of N-aryl compounds. Recovery tests confirm that the catalyst can be easily recovered and reused up to eight times without significant loss of its catalytic activity.
Introduction
In the past several years, ‘Green Chemistry’ has become one of the hottest terms in chemistry. If we relate the coupling reactions and the 12 principles of Green Chemistry,1 the use of a green solvent and non-toxic catalyst are the most obvious aspects we can consider. Concerning the green solvent, water is the most obvious candidate, because it is the cheapest and safest solvent and is non-toxic.2 Regarding the green catalyst, superparamagnetic nanoparticles (SMNPs) have attracted much attention because of their low toxicity, large ratio of surface area to volume, superparamagnetic behaviour, convenient surface modification, and their easy separation from a reaction mixture.3 Besides the easy separation, the small size of the magnetic nanoparticles (typically <50 nm) make them highly dispersible in solvents. Utilizing these advantages of magnetic nanoparticles over other supporting materials, various catalysts and ligands have been immobilized on these particles.4Aromatic amines have attracted considerable attention because of their significant biological activities, and they are used in a range of applications, from natural products to medicinal agents.5–7 Several synthetic and naturally occurring arylamines are known to act as scaffolds and show diverse biological activities (Scheme 1). For example, folic acid (vitamin B9) is well known to be essential for numerous bodily functions,8 teleocidins [ex. teleocidin B-1], olivoretins [ex. olivoretin A], and lyngbyatoxins [ex. lyngbyatoxin B], and indole alkaloids with an arylamine moiety have been isolated from microorganisms (Streptomyces and Streptoverticillium) and blue-green alga (Lyngbya species) and used as strong tumour promoters. Benzolactam V8, an artificially designed cyclic dipeptide derived from an arylamine, is a protein kinase C inhibitor.9
An essential methodology for the preparation of aromatic amines is based on the C–N cross-coupling reaction. Ullmann10 and Goldberg11 for the first time used copper for the C–N cross-coupling reaction. However, the synthetic scope of their reaction is strongly limited by the high reaction temperatures required (200 °C) and the sensitivity of the substituted aryl halide to the harsh reaction conditions applied. Since copper can easily access Cu(0), Cu(I), Cu(II), and Cu(III) oxidation states, allowing it to act through one-electron or two-electron processes,12 a number of research groups have examined the utility of a copper catalyst in the synthetic method for C–N coupling reactions.
Breakthroughs in copper-catalysed cross-coupling reactions have been driven independently by Lam,13 Chan,14 and Evans15 in 1998, who demonstrated that C–N bond formation could be rendered by the use of arylboronic acids as arylating agents instead of aryl halides under milder conditions in the presence of more than an equimolar amount of Cu(OAc)2. Though dramatic progress has been made with regards to N-arylation using arylboronic acids,16 little progress has been made to develop green copper catalysts for this reaction. Recently though, copper catalysts supported on the resin,17,18 chloromethyl polystyrene,19 cellulose,20 and fluorapatite21 have all been used as recyclable catalysts in heterogeneous systems to promote the copper-catalyzed C–N coupling reaction. However, despite these important contributions, more work still needs to be done to identify new supports and hence the search for a highly effective catalyst system is still challenging and interesting.
Recently, we used magnetic nanoparticles-supported Cu(II)-β-cyclodextrin complex for the synthesis of symmetrical biaryls and 1,2,3-triazoles.22 Herein, the aim of this work is to prepare and characterize a magnetically separable copper(II)–EDTA complex (Fe3O4-EDTA–Cu(II)), and to test its application as a filtration-free and recyclable heterogeneous catalyst for the synthesis of N-arylamines through the coupling reaction of aryl/alkyl amines with arylboronic acids in water under aerobic conditions.
Results and discussion
EDTA-functionalized magnetic nanoparticles (Fe3O4-EDTA–NMP) were prepared by the method described by Bahadur et al.23 with some modifications. The Fe3O4-EDTA nanoparticles were treated with aqueous CuSO4 solution at room temperature for 1 h to provide Fe3O4-EDTA–Cu(II) nanoparticles (Scheme 2). The structure of the prepared catalyst was characterized by various spectroscopic analyses, including FT-IR, TGA, SEM, TEM, VSM, and AAS.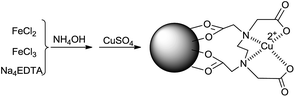 | ||
| Scheme 2 Possible structure and preparation method of the Fe3O4-supported EDTA–Cu(II) nanoparticles. | ||
Fig. 1 shows the FT-IR spectra (in the 400–4000 cm−1 wavenumber range) of the magnetic Fe3O4-EDTA–Cu(II) nanoparticles. It shows that the intense adsorption band of the Fe–O bonds in the tetrahedral sites is 585.9 cm−1. The broad band at 3000–3500 cm−1 is due to the hydroxy stretching vibration. The presence of peaks at 2917, 1626, and 1049 cm−1 are ascribed to the C–H stretching, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching, and the aliphatic C–N stretching vibration, respectively, indicating the existence of EDTA. The assignments are concordant with those of that Yang et al.24 and Liu et al. reported on Fe3O4-EDTA.25 Therefore, it can be concluded that EDTA was supported successfully on the surface of the Fe3O4 nanoparticles.
O stretching, and the aliphatic C–N stretching vibration, respectively, indicating the existence of EDTA. The assignments are concordant with those of that Yang et al.24 and Liu et al. reported on Fe3O4-EDTA.25 Therefore, it can be concluded that EDTA was supported successfully on the surface of the Fe3O4 nanoparticles.
The presence of EDTA on the surface of the magnetic nanoparticles was proved by thermogravimetric analysis (TGA) at a heating rate of 10 °C min−1 in the air over a temperature range of 25–600 °C (Fig. 2). The TGA curve of the Fe3O4-EDTA–Cu(II) nanoparticles shows a weight loss of 1.93% below 160 °C, which is due to the loss of the adsorbed water in the sample. The mass loss in the range of 200–400 °C is attributed to the thermal decomposition of EDTA. Accordingly, it is revealed that EDTA indeed is attached onto the surface of the Fe3O4 nanoparticles. The decomposition of EDTA on the Fe3O4-EDTA–Cu(II) nanoparticles at a lower temperature than pure EDTA shows the good interaction of EDTA with the Fe3O4 nanoparticles. In addition, TG analysis indicated that the prepared Fe3O4-EDTA–Cu(II) nanoparticles have high thermal stability and negligible EDTA leaching up to about 200 °C.
Scanning and transmission electron microscope (SEM and TEM) images were used to obtain direct information about the structure and morphology of the Fe3O4-EDTA–Cu(II) nanoparticles. The SEM image revealed that the Fe3O4-EDTA–Cu(II) particles are nearly spherical in shape and tend to agglomerate into larger aggregates (Fig. 3). TEM images of the Fe3O4 and Fe3O4-EDTA–Cu(II) nanoparticles are shown in Fig. 4. The TEM image of Fe3O4 particles clearly shows the formation of roughly spherical Fe3O4 nanoparticles, with a mean size range of 10–20 nm. The TEM image of the Fe3O4-EDTA–Cu(II) nanoparticles shows that the presence of EDTA during the production of the Fe3O4 nanoparticles leads to a decrease in the size of the nanoparticles (∼12 nm) and that the synthesized nanoparticles are nearly monodisperse. This reveals that EDTA plays a surfactant role to reduce the nanoparticle size. No detectable layer of EDTA can be observed on the TEM of the Fe3O4-EDTA–Cu(II) nanoparticles, which is due to the small amount of EDTA attached on the surface (∼3 wt% from the TG analysis).
It is known that magnetic particles with sizes less than about 30 nm with zero coercivity and zero remanence exhibit superparamagnetism.26,27 The magnetic properties of the Fe3O4 and Fe3O4-EDTA–Cu(II) nanoparticles were investigated using a vibrating sample magnetometer (VSM) at room temperature. Fig. 5 shows the VSM curves of the Fe3O4 (a), and Fe3O4-EDTA–Cu(II) particles (b), respectively in an applied magnetic field of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Oe at 298 K measured by the Meghnatis Daghigh Kavir Company (Iran). The hysteresis loops of the Fe3O4-EDTA–Cu(II) nanoparticles show superparamagnetic behaviour with their re-dispersion stability in solution without aggregation. The saturation magnetization of 16.3 emu g−1 is sufficient for magnetic separation of the magnetic nanoparticles with a conventional permanent magnet.28 The saturation magnetizations of the magnetic Fe3O4 and Fe3O4-EDTA–Cu(II) nanoparticles were found to be 65.58 emu g−1 and 58.75 emu g−1, respectively. These values are high enough to separate magnetic Fe3O4 and Fe3O4-EDTA–Cu(II) nanoparticles from aqueous solution (Fig. 6).
000 Oe at 298 K measured by the Meghnatis Daghigh Kavir Company (Iran). The hysteresis loops of the Fe3O4-EDTA–Cu(II) nanoparticles show superparamagnetic behaviour with their re-dispersion stability in solution without aggregation. The saturation magnetization of 16.3 emu g−1 is sufficient for magnetic separation of the magnetic nanoparticles with a conventional permanent magnet.28 The saturation magnetizations of the magnetic Fe3O4 and Fe3O4-EDTA–Cu(II) nanoparticles were found to be 65.58 emu g−1 and 58.75 emu g−1, respectively. These values are high enough to separate magnetic Fe3O4 and Fe3O4-EDTA–Cu(II) nanoparticles from aqueous solution (Fig. 6).
Fig. 7 shows the XRD patterns of Fe3O4 and Fe3O4-EDTA–Cu(II) nanoparticles. The XRD patterns of Fe3O4 (Fig. 7a) and Fe3O4-EDTA–Cu(II) nanoparticles (Fig. 7b) indicate the formation of a single phase magnetite with lattice constant, a = 8.374 Å (JCPDS Card no. 88-0315, a = 8.375 Å). Six characteristic peaks at 2θ = 30.3°, 35.7°, 43.4°, 53.9°, 57.8°, and 63.1° were simultaneously found in all the XRD patterns of Fe3O4 and Fe3O4-EDTA–Cu(II) nanoparticles, which related to the (220), (311), (400), (422), (511), and (440) phases of Fe3O4, respectively. This disclosed that the resultant nanoparticles were pure Fe3O4 with a spinel cubic structure, and that the supporting of the surface of Fe3O4 nanoparticles with EDTA–Cu(II) complex did not result in the phase change of Fe3O4. The XRD results also demonstrate the high crystallinity of prepared magnetic nanoparticles. The crystal size of the magnetic Fe3O4 and Fe3O4-EDTA–Cu(II) nanoparticles were determined from the XRD pattern using Scherrer's equation.29 The values provided for the magnetic Fe3O4 and Fe3O4-EDTA–Cu(II) nanoparticles by the above equation were 28 nm and 14 nm, respectively. The amount of copper (1%) needed for the Fe3O4-EDTA–Cu(II) nanoparticles was determined by atomic absorption analysis (AAS).
The catalytic behaviour of the prepared Fe3O4-EDTA–Cu(II) nanoparticles was studies in the cross-coupling reaction of amines with arylboronic acids in water as a green solvent. The efficiency of the cross-coupling of amines with arylboronic acids in water may be hampered by the side reactions of boronic acids themselves, such as the homo-coupling reaction, or the hydrolytic deboronation with water. Therefore, the cross-coupling of aniline (1a) with phenylboronic acid (2a) was initially chosen as the model reaction. The results are shown in Table 1.
| Entry | Catalyst | Temp (°C) | t (h) | Yielda (%) |
|---|---|---|---|---|
| a GC yields. | ||||
| 1 | None | r.t. | 12 | N.R |
| 2 | Fe3O4 (100 mg) | r.t. | 12 | N.R |
| 3 | Fe3O4-EDTA (100 mg) | r.t. | 12 | N.R |
| 4 | EDTA–Cu(II)(10 mol%) | r.t. | 5 | 85 |
| 5 | Fe3O4-EDTA–Cu(II) (5 mol%) | r.t. | 8 | 95 |
| 6 | Fe3O4-EDTA–Cu(II) (10 mol%) | r.t. | 5 | 86 |
| 7 | Fe3O4-EDTA–Cu(II) (15 mol%) | r.t. | 3 | 71 |
| 8 | Fe3O4-EDTA–Cu(II) (5 mol%) | 50 | 2 | 95 |
No reaction occurred in the absence of the copper catalyst (entry 1). A control experiment also showed that both reactions with Fe3O4 and Fe3O4-EDTA nanoparticles did not occur (entries 2 and 3). When the reaction was carried out with 10 mol% of the EDTA–Cu(II) complex and the Fe3O4-EDTA–Cu(II) catalyst at room temperature (entries 4 and 6) in water for 5 h, biphenylamine (3a) was obtained in 85% and 86% yields, as expected. After evaluation of the catalytic amounts, the reaction time, and the reaction temperature (entries 5–8), the best result was obtained from the conditions at 50 °C for 2 h using 5 mol% of the catalyst in air (entry 8).
To survey the generality of this C–N coupling reaction, the scope and limitations of this protocol were examined using a series of primary aromatic and aliphatic amines, along with amide derivatives, under optimized reaction conditions (Table 2). In the cross-coupling of aniline derivatives containing various substituents with phenylboronic acid, the corresponding N-phenylated products were obtained in excellent to good yields (Table 2, entries 1–6). para-Substituted anilines were found to be better substrates than ortho-substituted analogues (Table 2, entries 2, 4 vs. 3, 5). These results show that the coupling reaction was slightly affected by steric congestion around the amino functionality of the aniline derivatives. No significant steric effect was observed with the reaction of 2-methylphenylboronic acid (entry 7 vs. 8). N-Dealkylation has been reported to be a potentially serious side reaction in the N-arylation of aliphatic amines using arylboronic acids.30 The cross-coupling of primary aliphatic amines produced the corresponding N-arylamines in good to excellent yields (Table 2, entries 10–16) and no trace of the diarylated, alkyldiphenylamine or N-dealkylated (aniline or diphenylamine) by-products were detected. Aliphatic amines bearing olefin moieties and cyclohexylamine as a branched amine also gave the corresponding amine products in good yield, while the reaction with isopropylamine gave only phenol as a product (Table 2, entries 14, 15, 19). Moreover, the cross-coupling of toluenesulfonamide with phenylboronic acid was also accomplished with a high yield under the same conditions (Table 2, entry 17). We then applied this method to amides, including benzamide, but the cross-coupling reaction failed and only the phenol product was detected.
| Entry | Amine | Boronic acid | Product3 | t (h) | Yielda (%) |
|---|---|---|---|---|---|
| a Isolated yields.b Only phenol was detected. | |||||
| 1 | C6H5– | 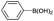 |
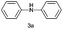 |
2 | 95 |
| 2 | p-MeC6H4– |  |
 |
2 | 95 |
| 3 | o-MeC6H4– |  |
 |
2 | 93 |
| 4 | p-MeOC6H4– |  |
 |
2 | 99 |
| 5 | o-MeOC6H4– | 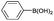 |
 |
2 | 89 |
| 6 | m-HOC6H4– |  |
 |
2 | 96 |
| 7 | C6H5– |  |
 |
1 | 96 |
| 8 | C6H5– |  |
 |
1 | 93 |
| 9 | C6H5– |  |
 |
2 | 96 |
| 10 | n-Pr– |  |
 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 45 |
43 |
| 11 | n-Bu– |  |
 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
83 |
| 12 | n-Hex– |  |
 |
1 | 80 |
| 13 | n-Oct– |  |
 |
1 | 97 |
| 14 | C6H11– |  |
 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
85 |
| 15 | Allyl– |  |
 |
4 | 35 |
| 16 | HO–CH2CH2– |  |
 |
2 | 96 |
| 17 | Ts– |  |
 |
1 | 96 |
| 18 | C6H5CO– |  |
 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
—b |
| 19 | i-Pr | 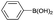 |
 |
2 | —b |
The coupling reaction of ethanol amine and 3-aminophenol with phenylboronic acid gave the corresponding N-arylamines in a high yield, without any C–O cross-coupling product (Table 2, entries 6 and 16).
A comparison with other supported copper catalytic systems in the cross-coupling reaction of aniline with phenylboronic acids demonstrated that our present catalytic system exhibited a higher conversion and yield in green and milder conditions (Table 3).
| Entry | Catalyst | Reaction conditions | t (h) | Yield (%) | Reference |
|---|---|---|---|---|---|
| 1 | Wang resin supported | Cat 1.5 equiv., CH2Cl2, NEt3, r.t. | 24 | 66 | 18 |
| 2 | Resin-supported | Cat 10 mol%, CH2Cl2, 1 atm O2, r.t. | 24 | 32 | 17 |
| 3 | Cu-FAP | Cat 100 mg, MeOH, r.t. | 3 | 90 | 21 |
| 4 | Polymer supported | Cat 2.6 mol%, DMSO, 140 °C, K2CO3 | 12 | 86 | 19 |
| 5 | Fe3O4-EDTA–Cu(II) | 5 mol%, H2O, 50 °C | 2 | 95 | This study |
Control experiments using CuCl2, CuSO4, and CuI as a catalyst also revealed the yields of N-arylation product in the coupling reaction of aniline with 4-methylephenylboronic acid in water at 50 °C were very low and a homo-coupling product (4,4′-dimethyl-1,1′-biphenyl) was detected as a by-product (Table 4).
| Entry | Catalyst | Yield (%) |
|---|---|---|
| 1 | CuI | 10 |
| 2 | CuCl2 | 25 |
| 3 | CuSO4 | 16 |
| 4 | Fe3O4-EDTA–Cu(II) | 98 |
For a supported catalyst, the lifetime and recycling of the catalyst are important issues to be resolved. To address these issues, we turned our attention to the reusability of Fe3O4-EDTA–Cu(II) nanoparticles in the N-arylation reaction of aniline with 4-methylephenylboronic acid under the same reaction conditions. After carrying out the reaction, the catalyst was collected by a magnet and washed with deionized water and acetone and reused without further purification. From the results, it was shown that the catalyst retained its high catalytic activity over eight repeating cycles (Fig. 8). To confirm whether the un-leached copper catalyst in the solution promoted the cross-coupling reaction, the following experiment was conducted. After suspending the Fe3O4-EDTA–Cu(II) nanoparticles in water overnight and then filtering off the catalyst, the cross-coupling reaction of aniline and 4-methylephenylboronic acid was performed in the filtrate. After 12 h, no conversion was detected following gas chromatography analysis (GC). An atomic absorption analysis also showed that there was no leaching of the copper ion from the catalyst after ten cycles. That there was no leaching of copper after ten cycles showed that this method can be used as a powerful method in pharmaceutical applications.
A possible mechanism for N-arylation is outlined in Scheme 3. We believe that a copper(I)/copper(III) catalytic system is in place, because a reductive elimination of a copper(II) intermediate to copper(0) would dissociate the copper from the catalyst, rendering it non-recyclable. The mechanism is based on those proposed by Collman,31 Lam,32 and Demir.33 Initially, coordination of the amine to Fe3O4-EDTA–Cu(II) and oxidation by oxygen gives a copper(III) intermediate 4. The transmetalation of 4 with the arylboronic acid gives 5, which undergoes reductive elimination to give product 7, and also provides the copper(I)-bound catalyst 6. Coordination of the amine and oxidation by oxygen leads to regeneration of the copper(III) intermediate 4.
Conclusion
In conclusion, we developed a rapid, efficient, and convenient protocol for the preparation of N-arylated amines by the coupling of arylboronic acids with amines in the presence of Fe3O4-EDTA–Cu(II) catalyst. Our inexpensive catalytic system showed a great functional group tolerance in the presence of multiple potentially reactive groups at moderate temperature. The short reaction time and simple reaction conditions render this method particularly attractive for the efficient construction of library synthesis and for the preparation of biologically and medicinally interesting molecules. Furthermore, the Fe3O4-EDTA–Cu(II) catalyst can be collected easily by a magnet and reused, with the reusability of the prepared nanocatalyst being successfully tested eight times with only a very slight loss of catalytic activity.Experimental
General method
All melting points were taken on a Yanagimoto and Buchi 510 apparatus and are uncorrected. Mass spectra were recorded on a VG Auto Spec. using electron impact ionization (EI) techniques. NMR spectra were obtained on a Bruker Avance 400 NMR Spectrometer (1H NMR: 400 MHz, 13C NMR: 100 MHz). Gas chromatography was performed on a Varian CP 3800 chromatograph. Analytical TLC was carried out with Merck plates pre-coated with silica gel 60 F254 (0.25 mm thick). Column chromatography was performed with a FLUKA Silica gel 60 (70–230 mesh) in common glass columns. Copper sulfate was recrystallized before use. Arylboronic acids, amines, and Na4EDTA were used as-received. All solvents were distilled before use.Preparation of Fe3O4-EDTA nanoparticles
EDTA-functionalized Fe3O4 were prepared by the co-precipitation method, followed by the in situ grafting of EDTA. Briefly, FeCl3·6H2O (2.36 g) and FeCl2·4H2O (0.86 g) were stirred to dissolve in distilled water (40 mL) at 90 °C under a N2 atmosphere. Subsequently, 20 mL ammonia solution (25%) was added dropwise to the reaction mixture under vigorous stirring. An aqueous solution containing Na4EDTA (5 g in 10 mL water) was added and the reaction mixture was stirred for 60 min. Finally, the temperature was cooled to room temperature and the prepared nanoparticles were collected by a permanent magnet and washed with distilled water repeatedly, and finally dried for 24 h at room temperature in a vacuum oven.Preparation of Fe3O4-EDTA–Cu(II) nanoparticles
Fe3O4-EDTA nanoparticles (1 g) were dispersed in distilled water (20 mL) containing CuSO4·5H2O (100 mg) and the mixture was stirred at room temperature. After 1 h, the nanoparticles were collected using an external magnet and washed repeatedly with distilled water and finally dried at room temperature in a vacuum oven.General procedure for the C–N coupling of arylboronic acid with amines
To a mixture of Fe3O4-EDTA–Cu(II) (0.05 mmol) and amine (3 mmol) in distilled water (2 mL), arylboronic acid (1 mmol) was added and the resultant mixture was stirred at 50 °C for 1–4 h. Then, the reaction mixture was diluted with water (20 mL), extracted with dichloromethane (2 × 20), and the combined extracts were dried with Na2SO4. The product was purified immediately by flash chromatography (silica gel 60; particle size 230–400 mesh; n-hexane/EtOAc) to afford arylamines 3a–n.Recyclability of Fe3O4-EDTA–Cu(II) catalyst
After carrying out the reaction, the catalyst was collected by a magnet and washed with deionized water and acetone and reused directly without further purification.References
- (a) P. Anastas and J. Warner, Green Chemistry: Theory and Practice, Oxford University Press, New York, 1998 Search PubMed; (b) P. Anastas and N. Eghbali, Chem. Soc. Rev., 2012, 39, 301–312 RSC.
- M. O. Simon and C. J. Li, Chem. Soc. Rev., 2012, 41, 1415–1427 RSC.
- (a) A. H. Lu, E. L. Salabas and S. Ferdi, Angew. Chem., Int. Ed., 2007, 46, 1222–1244 CrossRef CAS PubMed; (b) W. Schaertl, Nanoscale, 2010, 2, 829–843 RSC; (c) S. Sankaranarayanapillai, S. Volker and R. T. Werner, Angew. Chem., Int. Ed., 2010, 49, 3428–3459 CrossRef PubMed; (d) S. Laurent, D. Forge, M. Port, A. Roch, C. Robic, L. V. Elst and R. N. Muller, Chem. Rev., 2008, 108, 2064–2110 CrossRef CAS PubMed.
- V. Polshettiwar, R. Luque, A. Fihri, H. Zhu, M. Bouhrara and J. M. Basset, Chem. Rev., 2011, 111, 3036–3075 CrossRef CAS PubMed.
- M. L. Quan, P. Y. S. Lam, Q. Han, D. J. P. Pinto, M. Y. He, R. Li, C. D. Ellis, C. G. Clark, C. A. Teleha, J. H. Sun, R. S. Alexander, S. Bai, J. M. Luettgen, R. M. Knabb, P. C. Wong and P. R. Wexler, J. Med. Chem., 2005, 48, 1729–1744 CrossRef CAS PubMed.
- B. K. Singh, V. S. Christian, R. J. A. Davy, S. P. Virinder and V. V. E. Erik, Tetrahedron Lett., 2009, 50, 15–18 CrossRef CAS PubMed.
- N. Yamazaki, I. Washio, Y. Shibasaki and M. M. Ueda, Org. Lett., 2006, 8, 2321–2324 CrossRef CAS PubMed.
- (a) S. Gilbody, S. Lewis and T. Lightfoot, Am. J. Epidemiol., 2007, 165, 1–13 CrossRef PubMed; (b) M. J. Taylor, S. M. Carney, G. M. Goodwin and J. R. Geddes, J. Psychopharmacol., 2004, 18, 251–256 CrossRef CAS PubMed; (c) C. Keshava, N. Keshava, W. Z. Whong, J. Nath and T. M. Ong, Mutat. Res., 1998, 397, 221–228 CrossRef CAS.
- (a) M. Takashima and H. Sakai, Bull. Agric. Chem. Soc. Jpn., 1960, 24, 647–651 CrossRef CAS; (b) M. Takashima and H. Sakai, Bull. Agric. Chem. Soc. Jpn., 1960, 24, 652–655 CrossRef CAS; (c) H. Nakata, H. Harada and Y. Hirata, Tetrahedron Lett., 1966, 7, 2515–2522 CrossRef; (d) Y. Endo, K. Shudo, A. Itai, M. Hasegawa and S.-I. Sakai, Tetrahedron, 1986, 42, 5905–5924 CrossRef CAS; (e) J. H. Cardellina, F.-J. Marner and R. E. Moore, Science, 1979, 204, 193–195 CAS; (f) Y. Hitotsuyanagi, K. Yamaguchi, K. Ogata, N. Aimi, S.-I. Sakai, Y. Koyama, Y. Endo, K. Shudo, A. Itai and Y. Iitaka, Chem. Pharm. Bull., 1984, 32, 3774–3778 CrossRef CAS.
- F. Ullmann, Ber. Dtsch. Chem. Ges., 1903, 36, 2389–2391 Search PubMed.
- I. Goldberg, Ber. Dtsch. Chem. Ges., 1906, 39, 1691–1692 CrossRef.
- (a) E. A. Scott, R. R. Walvoord, P. S. Rosaura and C. K. Marisa, Chem. Rev., 2013, 113, 6234–6458 CrossRef PubMed; (b) J. Bariwalab and E. V. Eycken, Chem. Soc. Rev., 2013, 42, 9283–9303 RSC; (c) G. Evano, N. Blanchard and M. Toumi, Chem. Rev., 2008, 108, 3054–3131 CrossRef CAS PubMed.
- P. Y. S. Lam, C. G. Clark, S. Saubern, J. Adams, M. P. Winters, D. M. T. Chan and A. Combs, Tetrahedron Lett., 1998, 39, 2941–2944 CrossRef CAS.
- D. M. T. Chan, K. L. Monaco, R. P. Wang and M. P. Winters, Tetrahedron Lett., 1998, 39, 2933–2936 CrossRef CAS.
- D. A. Evans, J. L. Katz and T. R. West, Tetrahedron Lett., 1998, 39, 2937–2940 CrossRef CAS.
- For example: (a) B. Kaboudin, Y. Abedi and T. Yokomatsu, Eur. J. Org. Chem., 2011, 6656–6662 CrossRef CAS; (b) M. Nobis and B. Driessen-Holscher, Angew. Chem., Int. Ed., 2001, 40, 3983–3985 CrossRef CAS; (c) D. Hollmann, S. Bahn, A. Tillack and M. Beller, Angew. Chem., Int. Ed., 2007, 46, 8291–8294 CrossRef CAS PubMed; (d) G. C. Kobeci and J. M. J. Williams, Chem. Commun., 2004, 1072–1073 RSC; (e) M. Larrosa, C. Guerrero, R. Rodriguez and J. Cruces, Synthesis, 2010, 2101–2105 CAS.
- A. Biffis, F. Filippi, G. Palma, S. Lora, C. Maccàa and B. Corain, J. Mol. Catal. A: Chem., 2003, 203, 213–220 CrossRef CAS.
- C. H. G. Chiang and T. Olsson, Org. Lett., 2004, 6, 3079–3082 CrossRef PubMed.
- S. M. Islam, S. Mondal, P. Mondal, A. S. Roy, K. Tuhina, N. Salam and M. Mobarak, J. Organomet. Chem., 2012, 696, 4264–4274 CrossRef CAS PubMed.
- K. R. Reddy, N. S. Kumar, B. Sreedhar and M. L. Kantam, J. Mol. Catal. A: Chem., 2006, 252, 136–150 CrossRef CAS PubMed.
- M. L. Kantam, G. T. Venkanna, C. Sridhar, B. Sreedhar and M. B. Choudary, J. Org. Chem., 2006, 71, 9522–9524 CrossRef CAS PubMed.
- B. Kaboudin, R. Mostafalu and T. Yokomatsu, Green Chem., 2013, 15, 2266–2274 RSC.
- S. Singh, K. C. Barick and D. Bahadur, AIP Conf. Proc., 2013, 1512, 440–441 CrossRef CAS PubMed.
- J. Yang, Q. Zeng, L. Peng, M. Lei, H. Song, B. Tie and J. Gu, J. Environ. Sci., 2013, 25, 413–418 CrossRef CAS.
- Y. Liu, M. Chen and Y. Hao, Chem. Eng. J., 2013, 218, 46–54 CrossRef CAS PubMed.
- B. Li, D. Jia, Y. Zhou, Q. Hu and W. Cai, J. Magn. Magn. Mater., 2006, 306, 223–227 CrossRef CAS PubMed.
- S. M. O'Brien, O. Thomas and P. Dunnill, J. Biotechnol., 1996, 50, 13–26 CrossRef.
- Z. Y. Ma, Y. P. Guan and H. Z. Liu, J. Polym. Sci., Part A: Polym. Chem., 2005, 43, 3433–3439 CrossRef CAS.
- A. Z. M. Badruddoza, G. S. S. Hazel, K. Hidajat and M. S. Uddin, Colloids Surf., A, 2010, 367, 85–95 CrossRef CAS PubMed.
- Y. Liu, M. Chen and Y. Hao, Chem. Eng. J., 2013, 218, 46–54 CrossRef CAS PubMed.
- (a) J. P. Collman and M. Zhong, Org. Lett., 2000, 2, 1233–1236 CrossRef CAS; (b) J. P. Collman, M. Zhong, L. Zeng and S. Costanzo, J. Org. Chem., 2001, 66, 1528–1531 CrossRef CAS.
- J. C. Antilla and S. L. Buchwald, Org. Lett., 2001, 3, 2077–2079 CrossRef CAS PubMed.
- P. Y. S. Lam, G. Vincent, C. G. Clark, S. Deudon and P. K. Jadhav, Tetrahedron Lett., 2001, 42, 3415–3418 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See%20DOI:%2010.1039/c4ra08137d |
| This journal is © The Royal Society of Chemistry 2014 |












