Continuous flow synthesis of β-amino acids from α-amino acids via Arndt–Eistert homologation†
Abstract
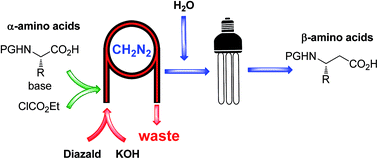
A fully continuous four step process for the preparation of β-amino acids from their corresponding α-amino acids utilizing the Arndt–Eistert homologation approach is described.
- This article is part of the themed collection: Recent Advances in Flow Synthesis and Continuous Processing

 Please wait while we load your content...
Please wait while we load your content...