A comparative study on binding ability of three lanthanide ions with centrin using impedance method†
Zhijiang Rongab,
Yanni Tiana and
Binsheng Yang*a
aInstitute of Molecular Science, Key Laboratory of Chemical Biology of Molecular Engineering of Education Ministry, Shanxi University, Taiyuan 030006, PR China
bSchool of Environment and Safety, Taiyuan University of Science and Technology, Taiyuan, 030024, PR China. E-mail: yangbs@sxu.edu.cn; Tel: +86-351-7016358
First published on 20th August 2014
Abstract
Centrin, an EF-hand calcium-binding protein, is involved in the formation of the microtubule organizing center (MTOC). The interactions between Gd3+, Eu3+, La3+ and the N-terminal domain of ciliate Euplotes octocarinatus centrin (N-EoCen) were investigated by electrochemical impedance spectroscopy (EIS) using potassium ferricyanide as a redox probe. Results show that an increase in the lanthanide ion (Ln3+) concentration resulted in a lower impedance magnitude in the Nyquist plots when the N-EoCen-modified glassy carbon electrode (N-EoCen-GC) was used. The titrations of N-EoCen with Ln3+ confirm the complexation reactivity of Ln3+ with N-EoCen and suggest that the Ln2–N-EoCen complex was formed. In contrast, the binding ability of Ca2+ to N-EoCen is far less than that of Ln3+. The binding constants of the metal ions to N-EoCen are in the order of Gd3+ ≈ Eu3+ > La3+ ≫ Ca2+. Compared with the resonance light scattering (RLS) method, the EIS method can not only distinguish the binding ability of different metal ions to N-EoCen, but can also distinguish clearly the different metal ion binding sites on N-EoCen.
1 Introduction
Centrin is a kind of low-molecular-weight acidic protein (about 20 kD). It belongs to the highly conservative EF-hand calcium-binding protein family. It plays an important role in centrosome orientation, mitosis and formation of the spindle and microtubule.1–3 One important function of centrin is fiber contraction, which is calcium-dependent and closely related to the aggregation of centrin.4–6 EoCen consists of two independent domains tethered by a flexible linker, each domain comprising a pair of EF-hand helix-loop-helix motifs that can potentially bind two calcium ions.7Rare earth ions are widely used in rare earth micronutrient fertilizers in agriculture. They are important in the research on combinations between rare earth ions and various types of protein. In the past few years, our group has been studying the interaction between rare earth ions and centrin using methods such as fluorescence, CD spectra and polyacrylamide gel electrophoresis.8–12 Rare earth ions can be used as a probe for calcium ions, which are spectrally silent, as their coordination chemistry properties are similar to those of calcium and they have a similar ionic radius. Ln3+, including La3+, Tb3+, and Lu3+, can compete with Ca2+ at calcium-binding sites. The combination of Ln3+ with the four binding sites of EoCen is orderly, similar to that with Ca2+.13 It has been reported that aggregation is closely related to the biological functions of EoCen, such as initiation of flagellar excision and fiber contraction. Ln3+ can induce the aggregation of EoCen, and the degree of aggregation induced by Lu3+ is about 5 times that with Ca2+. In addition, the importance of the N-terminal domain in the process of EoCen self-association has been proved.10 In the light of our previous work, the order of metal ion-induced conformational change of EoCen is Ca2+ < La3+ < Tb3+ < Lu3+.
Sensitized fluorescence is a necessary signal in monitoring the combination of Ln3+ and EoCen when using fluorescence spectrometry. However, as the fluorescence of EoCen cannot be greatly sensitized by most Ln3+ ions, this causes some limitations in using fluorescence methods. Recently, our group established a new electrochemical method. Using a cyclic voltammetry (CV) method, the interactions of Eu3+ with N-EoCen were studied.14
Compared with the CV technique, EIS can not only give the exact impedance data, but also reflects the nature of the interface directly. Here, we prepared a truncated ciliate EoCen, N-EoCen, including the first (binding site I) and second (binding site II) EF-hand domains. The combinational properties between Ca2+, La3+, Eu3+ and Gd3+ with N-EoCen were explored using EIS, CV and RLS. The mechanism by which the response of CV or EIS varies with the Ln3+ ion has also been investigated. The effect of ionic strength on the adsorption of N-EoCen was examined.
2 Experimental
2.1 Materials
A truncated ciliate EoCen, N-EoCen, including the first and second EF-hand domains, was expressed and purified as described earlier.12 N-2-Hydroxyethylpiperazine-N-2-ethanesulfonic acid (Hepes) was purchased from Sigma and used without further purification. La2O3, Gd2O3, and Eu2O3 are 99.99% and purchased from Hunan, China. All other chemicals are of analytical grade. The Gd3+, Eu3+ and La3+ solution was prepared by dissolving the appropriate mass of the Gd2O3, Eu2O3 or La2O3 in hydrochloric acid, which was then standardized with EDTA in 0.1 M HAc–NaAc buffer at pH 5.5. Potassium ferricyanide as the probe reagent was purchased from Shanghai xin yu biological technology Co., Ltd.2.2 Electrochemical study
A CHI 660C electrochemical analyzer (Chen Hua Instrumental Co., Shanghai), in connection with a glassy carbon working electrode (GCE, Ø = 3 mm), a saturated calomel reference electrode (SCE) and a platinum wire auxiliary electrode, was used for the electrochemical measurement. The GCE surface was freshly polished to a mirror by polishing with an alumina–water slurry (high-purity Al2O3, particle size 0.3 and 0.05 μm, BDH) and sonicated briefly, followed by thorough rinsing with water. The electrochemical impedance was tested employing a solution of 1.0 mM K3Fe(CN)6 containing different concentrations of KCl. The frequency range was from 10 mHz to 100 kHz, the DC potential was the average potential (0.175 V) of the oxidation and reduction peaks, and the amplitude was 10 mV. Impedance data were fitted to the appropriate model using the ZSimpWin software (Ametek). The potential sweep rate used was 50 mV s−1 in CV experiments, using a potential window from 0.5 to −0.2 V.2.3 Resonance light scattering
Resonance light scattering (RLS) of samples was monitored by fluorescence in quartz cells of 1 cm optical path at 25 °C. The RLS was performed in 0.01 M Hepes at pH 7.4, 0.02 M KCl with a fluorescence spectrometer (F-2500, Hitachi, Japan) using the same excitation and emission wavelengths. Samples were prepared by gradually adding Ln3+ into solutions of proteins. An equilibrium time of 5 min was used between each titration.3 Results and discussion
3.1 Effect of ionic strength on the adsorption of N-EoCen
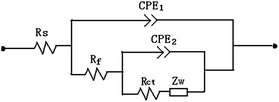
The adsorption of N-EoCen has been found to follow a Langmuir adsorption isotherm.14 Many electrochemical studies have been performed to evaluate the adsorption behavior of biomolecules. It has been shown that adsorption behavior is significantly affected by the ionic strength.15–17 However, it is unclear what the effect of ionic strength is on the adsorption of N-EoCen.The impedance under different ionic strengths was monitored versus the concentration of N-EoCen in 10 mM Hepes solutions (pH 7.4). Fig. 1A–D shows the Nyquist plots at a GC electrode using 1.0 mM potassium ferricyanide as probe ions in Hepes solutions containing 0.02, 0.04, 0.07 and 0.1 M KCl, respectively. The impedance results of Fig. 1 were fitted to the equivalent circuit (see Fig. 3), which is characteristic of an insulating layer over a conducting surface18–22 and yielded the best fit among several other circuits (solid line in Fig. 1).
Adsorption of N-EoCen onto the electrode surface can be described by the Langmuir isotherm equation.23–26
 | (1) |
The Langmuir isotherm equation can be rewritten as follows:
 | (2) |
 | (3) |
If the Langmuir isotherm is valid for an observed system, a plot of C/Rct versus concentration C should yield a straight line with the parameters Rmax and BADS derived from the slope and intercept, respectively.
The dependence of C/Rct versus C in Hepes buffer containing different concentrations of KCl is shown in Fig. 2A. It shows that a linear dependence exists in the range 0.02–0.1 M KCl. BADS is obtained. Then the plot of BADS versus concentration of KCl is shown in Fig. 2B. It can be seen that the affinity of the N-EoCen towards the GC electrode surface increases with decreasing concentration of KCl. The low ionic strength is beneficial for the adsorption of N-EoCen.
 | ||
| Fig. 2 (A): Langmuir adsorption isotherm presented in a linearized form according to eqn (3) for N-EoCen adsorbed onto the GC electrode in 0.01 M Hepes buffer solution (pH 7.4) in the presence of 1.0 mM Fe(CN)63−, containing different concentrations of KCl. (B): Dependence of BADS and the Gibbs energy (ΔGADS) of adsorption on the concentration of KCl for N-EoCen adsorbed onto the GC electrode. | ||
BADS can be presented by Gomma and Wahdan.27
 | (4) |
3.2 The interaction between La3+, Eu3+, Gd3+ and N-EoCen
EIS was used to study the interaction of the N-EoCen with Gd3+, Eu3+ and La3+. The N-EoCen was immobilized on the surface of the GC electrode according to the method described earlier.14 To obtain data about the impedance of modified electrodes, which allows tracking of the changes occurring at the interface, the Randles equivalent circuit shown in Fig. 3 was selected to fit the measured results. The changes in the charge transfer resistance (Rct) indicate the binding process of Ln3+ with N-EoCen. Examples of impedance spectra in relation to the concentration of Gd3+, La3+ and Eu3+ are shown in Fig. 4A–C, respectively. The measurements were performed in the electrolyte solution containing 0.02 M KCl, 0.01 M Hepes buffer (pH 7.4), and 1.0 mM [Fe(CN)6]3− at room temperature. It can be seen that the diameter of the Nyquist circles decreases with increasing concentration of Ln3+. It indicates that an increase in Ln3+ concentration resulted in a lower impedance magnitude in the Nyquist plots in the low-frequency section. Fig. 4D shows the dependence of Rct versus [Ln3+]/[N-EoCen].
It can be seen from Fig. 4D that Rct decreased slowly below 1.0 equiv. of Ln3+. However, Rct decreased rapidly with an additional 1.0 equiv. of Ln3+. It changed slowly above 2.0 equiv. of added Ln3+. The titration curve shows that the electron-transfer resistance varies with the concentration of added Ln3+, due to the combination of Ln3+ with N-EoCen. The first equivalent of added Ln3+ appears to be bound quantitatively with minimal response. A stronger electrochemical response was induced with the addition of another equivalent of Ln3+, which reached a final plateau at a [Ln3+]/[N-EoCen] ratio of up to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The titration curves reveal two breaks at [Ln3+]/[N-EoCen] = 1.0 and 2.0, confirming that a 2
1. The titration curves reveal two breaks at [Ln3+]/[N-EoCen] = 1.0 and 2.0, confirming that a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric ratio of the Ln2–N-EoCen complex was formed. It can be seen that there are two inequivalent changes in Rct with increasing concentration of Ln3+, corresponding to the two different binding sites in N-EoCen.
1 stoichiometric ratio of the Ln2–N-EoCen complex was formed. It can be seen that there are two inequivalent changes in Rct with increasing concentration of Ln3+, corresponding to the two different binding sites in N-EoCen.
In order to compare the results measured in the same conditions, the binding constants were calculated using a Langmuir isotherm. This approach has been successfully applied for the determination of binding constants between cations and crown ethers.32–34 The Langmuir isotherm assumes an equal binding energy for all binding sites.
In the experiment presented, the changes in Rct are related to the binding of Ln3+ to N-EoCen. As to the combination with the first binding site of N-EoCen, the relation between the occupied binding sites Θ and the change in Rct is as follows:
 | (5) |
For the second binding site of N-EoCen, the relation is as follows:
 | (6) |
In the case of the Langmuir isotherm, Θ can be related to the binding constant Ka according to the equation:35
 | (7) |
 | (8) |
Combination of eqn (5), (6) and (8) gives:
 | (9) |
 | (10) |
Eqn (9) and (10) have been applied for the calculation of the binding constants for Ln3+–N-EoCen complexes associated with the first and second binding sites.
The ratio [Rct(0) − Rct(i)]/[Rct(i) − Rct(1)] varies linearly with the concentration of Gd3+ in the range [Gd3+]/[N-EoCen] from 0 to 1.0 (Fig. 5A), and [Rct(1) − Rct(i)]/[Rct(i) − Rct(∞)] also varies linearly in the range from 1.0 to 2.0 (Fig. 5B). Therefore, the binding constants could be calculated from the slope. Similarly, the linear plots for La3+ (Fig. S1C and D†) and Eu3+ (Fig. S1A and B†) were further explored, and the binding constants were obtained.
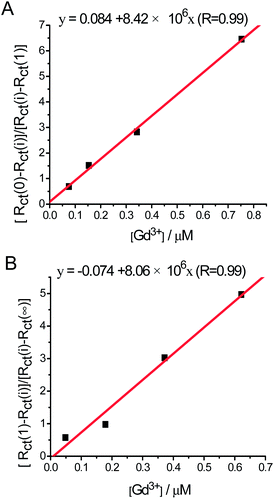 | ||
| Fig. 5 The linear relationship of [Rct(0) − Rct(i)]/[Rct(i) − Rct(1)] vs. [Gd3+] (A). The linear relationship of [Rct(1) − Rct(i)]/[Rct(i) − Rct(∞)] vs. [Gd3+] (B). | ||
The affinities of Gd3+, Eu3+ and La3+ towards binding site I of N-EoCen are (8.42 ± 0.27) × 106, (8.25 ± 0.97) × 106 and (5.21 ± 0.44) × 106 M−1, respectively, while the affinities of Gd3+, Eu3+ and La3+ towards binding site II of N-EoCen are (8.06 ± 0.78) × 106, (7.45 ± 0.55) × 106 and (2.75 ± 0.34) × 106 M−1. The ionic potentials for Ln3+ and the binding constants calculated are summarized in Table 1. On the whole, the affinity order of Ln3+ binding to N-EoCen is Gd3+ ≈ Eu3+ > La3+, which is consistent with the order of the Ln3+ ionic potentials (see Table 1).
| Metal ion | Gd3+ | Eu3+ | La3+ | Ca2+ |
|---|---|---|---|---|
| Ionic potential e/r | 0.0320 | 0.0316 | 0.0283 | 0.0202 |
| Binding constant KI/M−1 | (8.42 ± 0.27) × 106 | (8.25 ± 0.97) × 106 | (5.21 ± 0.44) × 106 | (7.35 ± 0.50) × 103 |
| Binding constant KII/M−1 | (8.06 ± 0.78) × 106 | (7.45 ± 0.55) × 106 | (2.75 ± 0.34) × 106 | (7.35 ± 0.50) × 103 |
We speculate that the Rct change with adding Ln3+ may be due to N-EoCen aggregation induced by Ln3+. This is because the ferricyanide probe molecules can more easily approach the vacant GC electrode surface resulting from the aggregation of N-EoCen.
EIS measurement can provide a reliable method to detect the interaction between the metal ions and the protein, and can further distinguish the binding abilities of different metal ions to N-EoCen. This measuring system is relatively simple in comparison to other spectroscopic methods. The spectroscopic method often needs to introduce a strong chromophore into N-EoCen, benefiting from the energy transfer between Ln3+ and chromophore residues.
The peak separations (ΔEp) of the redox reaction as a function of [Ln3+]/[N-EoCen] are presented in Fig. 6B. With an increasing concentration of Ln3+, the peak separation decreased. It can be seen from Fig. 6B that the peak separation decreased slightly below 1.0 equiv. of Ln3+, while with an additional 1.0 equiv. of Ln3+ the peak separation decreased rapidly, and then it changed slowly above 2.0 equiv. of Ln3+.
The titration curves (Fig. 6B) in the CV experiment show a similar shape to the curves from EIS (Fig. 4D), indicating that the results obtained from the CV and EIS experiments match each other well.
A series of fluorescence RLS measurements was conducted at different wavelengths between 250 and 650 nm. The aggregation of N-EoCen in the presence of Gd3+ was monitored (Fig. 7A). A titration curve of RLS versus [Gd3+]/[N-EoCen] was plotted from Fig. 7A, as shown in Fig. 7B. When the first 1.0 equiv. Gd3+ was added to the solution of N-EoCen, RLS increased clearly. When the additional Gd3+ was added, RLS was enhanced significantly and finally reached a larger amplitude at 2.0 equiv. Gd3+. RLS for the addition of La3+ and Eu3+ was also measured in our experiment and this shows a similar increasing tendency of RLS (Fig. S3A and B†). The titration curves for La3+ and Eu3+ are also shown in Fig. 7B. These RLS experiments show similar titration curves to those obtained from electrochemistry experiments (Fig. 4D and 6B), which implies that aggregation of N-EoCen induced by Ln3+ plays a critical role in electrochemical titration experiments. The aggregation degree of N-EoCen induced by Ln3+ also conforms to the order Gd3+ ≈ Eu3+ > La3+. This result is consistent with that obtained from EIS measurement.
In combination with the high similarity of the three types of experiment and the nature of aggregation reflected by RLS, this can provide valid evidence that the titration curve in electrochemical experiments reflects the aggregation property of N-EoCen to a certain extent.
In RLS titration curves, there is a break at [Ln3+]/[N-EoCen] = 1.0, which can be faintly observed. However, there is a clear break at [Ln3+]/[N-EoCen] = 1.0 in the EIS (Fig. 4D) and CV titration curves (Fig. 6B), suggesting that electrochemical methods can distinguish these two binding sites more easily than RLS experiments. Moreover, the RLS titration curves of La3+, Eu3+ and Gd3+ are very close. It is difficult to distinguish the aggregation degrees for these three Ln3+ clearly. However, due to the sensitivity of probe ions to protein–Ln3+ interaction at N-EoCen-GC, EIS and CV can distinguish the different binding abilities of Ln3+ to N-EoCen easily.
3.3 The interaction between Ca2+ and N-EoCen
Centrin is a calcium-binding protein. The combination of centrin and Ca2+ can cause protein aggregation and conformational change in centrin. Based on this characterization of Ca2+ binding, its natural biological function can be evaluated. Here, the interaction between Ca2+ and N-EoCen has also been investigated by CV. Fig. 8A shows the CVs of Fe(CN)63−/4− in 10 mM Hepes buffer (20 mM KCl) using a N-EoCen-GC electrode containing different concentrations of Ca2+. With an increasing concentration of Ca2+ the redox peak currents increase slowly. This implies that the N-EoCen immobilized on the GC electrode can react weakly with Ca2+.The peak separations (ΔEp) of the redox reaction as a function of [Ca2+]/[N-EoCen] were plotted (Fig. 8B). The titration curve shows that there is little change in CV when the concentration ratio of Ca2+ to N-EoCen is 1–10. Peak currents increase sharply and peak separations decrease clearly above [Ca2+]/[N-EoCen] ≧ 20. They change slowly for [Ca2+]/[N-EoCen] ratios up to 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. This indicates that Ca2+ combines with N-EoCen when excess Ca2+ is added. Compared with Fig. 6B, Ca2+ exhibits much lower binding ability to N-EoCen. This result is consistent with the conclusions reported.7,37 Due to the weak affinity of Ca2+ for N-EoCen, we can substitute the free concentration of Ca2+ with the total concentration of Ca2+ and further evaluate the average binding constant of Ca2+ to N-EoCen. KCa–N-EoCen = (7.35 ± 0.50) × 103 is obtained and also listed in Table 1. Then, the affinity order of metal ions to N-EoCen is Gd3+ ≈ Eu3+ > La3+ ≫ Ca2+, which is consistent with the ionic potential order (Table 1).
1. This indicates that Ca2+ combines with N-EoCen when excess Ca2+ is added. Compared with Fig. 6B, Ca2+ exhibits much lower binding ability to N-EoCen. This result is consistent with the conclusions reported.7,37 Due to the weak affinity of Ca2+ for N-EoCen, we can substitute the free concentration of Ca2+ with the total concentration of Ca2+ and further evaluate the average binding constant of Ca2+ to N-EoCen. KCa–N-EoCen = (7.35 ± 0.50) × 103 is obtained and also listed in Table 1. Then, the affinity order of metal ions to N-EoCen is Gd3+ ≈ Eu3+ > La3+ ≫ Ca2+, which is consistent with the ionic potential order (Table 1).
4 Conclusion
In this study, it is revealed that the adsorption of N-EoCen at a GC surface depends on ionic strength. With increasing ionic strength, the adsorbed amount decreased. This shows that electrostatic forces play an important role in the adsorption of N-EoCen. Currently, Ln3+ ion/N-EoCen interactions are investigated at the same ionic strength by EIS. Results show that the binding of Ln3+ to N-EoCen resulted in a lower impedance magnitude in the Nyquist plots. The Ln3+–N-EoCen complex obeys a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry, and the two binding sites of N-EoCen are not equivalent, from the titration curves. The affinity order is Gd3+ ≈ Eu3+ > La3+ ≫ Ca2+. EIS is a more sensitive method than RLS for differentiating the two binding sites of N-EoCen and the binding abilities of different Ln3+.
1 stoichiometry, and the two binding sites of N-EoCen are not equivalent, from the titration curves. The affinity order is Gd3+ ≈ Eu3+ > La3+ ≫ Ca2+. EIS is a more sensitive method than RLS for differentiating the two binding sites of N-EoCen and the binding abilities of different Ln3+.
Acknowledgements
This work was supported by the national natural science foundation of the People's Republic of China (Grant nos 20771068 and 20901048) and the PhD Programs Foundation of the Ministry of Education of China (Grant no. 20131401110011).Notes and references
- B. Huang, A. Mengersen and V. Lee, J. Cell Biol., 1988, 107, 13 Search PubMed.
- E. Schiebel and M. Bornens, Trends Cell Biol., 1995, 5, 197 CrossRef CAS.
- J. Salisbury, Curr. Opin. Cell Biol., 1995, 7, 39 CrossRef CAS.
- M. Tourbez, C. Firanescu, A. Yang, L. Unipan, P. Duchambon, Y. Blouquit and C. Craescu, J. Biol. Chem., 2004, 279, 47672 CrossRef CAS PubMed.
- J. Salisbury, A. Baron and M. Sanders, J. Cell Biol., 1988, 107, 635 CrossRef CAS.
- H. Wiech, B. M. Geier, T. Paschke, A. Spang, K. Grein, J. SteinkÖtter, M. Melkonian and E. Schiebel, J. Biol. Chem., 1996, 271, 22453 CrossRef CAS PubMed.
- C. Weber, V. D. Lee, W. J. Chazin and B. Huang, J. Biol. Chem., 1994, 269, 15795 CAS.
- L. X. Ren, Y. Q. Zhao, J. Y. Feng, X. J. He, A. H. Liang and B. S. Yang, Chin. J. Inorg. Chem., 2006, 22, 87 CAS.
- Z. J. Wang, Y. Q. Zhao, L. X. Ren, G. T. Li, A. H. Liang and B. S. Yang, J. Photochem. Photobiol., A, 2007, 186, 178 CrossRef CAS PubMed.
- L. Duan, Y. Q. Zhao, Z. J. Wang, G. T. Li, A. H. Liang and B. S. Yang, J. Inorg. Biochem., 2008, 102, 268 CrossRef CAS PubMed.
- Y. Q. Zhao, L. Song, A. H. Liang and B. S. Yang, J. Photochem. Photobiol., B, 2009, 95, 26 CrossRef CAS PubMed.
- Y. Q. Zhao, J. Yan, L. Song, Y. N. Feng, A. H. Liang and B. S. Yang, Spectrochim. Acta, Part A, 2012, 87, 163 CrossRef CAS PubMed.
- Y. Q. Zhao, J. Y. Feng, A. H. Liang and B. S. Yang, Spectrochim. Acta, Part A, 2009, 71, 1756 CrossRef PubMed.
- Z. J. Rong, Y. Q. Zhao, B. Liu, Y. N. Tian and B. S. Yang, J. Electroanal. Chem., 2013, 707, 102 CrossRef CAS PubMed.
- M. Zelić and M. Lovrić, J. Electroanal. Chem., 2003, 541, 67 CrossRef.
- A. Ranieri, G. Battistuzzi, M. Borsari, S. Casalini, C. Fontanesi, S. Monari, M. J. Siwek and M. Sola, J. Electroanal. Chem., 2009, 626, 123 CrossRef CAS PubMed.
- R. Garjonytea, A. Malinauskasa and L. Gortonb, Bioelectrochemistry, 2003, 61, 39 CrossRef.
- N. P. Cosman, K. Fatih and S. G. Roscoe, J. Electroanal. Chem., 2005, 574, 261 CrossRef CAS PubMed.
- S. Omanovic and S. G. Roscoe, J. Colloid Interface Sci., 2000, 227, 452 CrossRef CAS PubMed.
- Q. Mohsen, S. A. Fadlallah and N. S. ElShenawy, Int. J. Electrochem. Sci., 2012, 7, 4510 CAS.
- O. R. Cámaraa, L. B. Avalleb and F. Y. Olivaa, Electrochim. Acta, 2010, 55, 4519 CrossRef PubMed.
- F. B. Diniz and R. R. Ueta, Electrochim. Acta, 2004, 49, 4281 CrossRef CAS PubMed.
- D. C. Hansen, G. W. Luther and J. H. Waite, J. Colloid Interface Sci., 1994, 168, 206 CrossRef CAS.
- A. Ouerd, C. A. Dumont, B. Normand and S. Szunerits, Electrochim. Acta, 2008, 53, 4461 CrossRef CAS PubMed.
- J. L. Brash and P. T. Hove, J. Biomater. Sci. Polym. Ed., 1993, 4, 591 CrossRef CAS PubMed.
- A. Klinger, D. Steinberg, D. Kohavi and M. N. Sela, J. Biomed. Mater. Res., 1997, 36, 387 CrossRef CAS.
- G. K. Gomma and M. H. Wahdan, Mater. Chem. Phys., 1994, 39, 142 CrossRef CAS.
- N. Kazuhiro, S. Takaharu and I. Koreyoshi, J. Biosci. Bioeng., 2001, 91, 233 Search PubMed.
- M. Kleijn and W. Norde, Heterog. Chem. Rev., 1995, 2, 157 CAS.
- M. Wahlgren and T. Arnebrant, Trends Biotechnol., 1991, 9, 201 CrossRef CAS.
- F. Patolsky, B. Filanovsky and E. Katz, J. Phys. Chem. B, 1998, 102, 10359 CrossRef CAS.
- S. Flink, B. A. Boukamp, A. Berg, F. C. J. M. Veggel and D. N. Reinhoudt, J. Am. Chem. Soc., 1998, 120, 4652 CrossRef CAS.
- S. Flink, F. C. J. M. Veggel and D. N. Reinhoudt, J. Phys. Chem. B, 1999, 103, 6515 CrossRef CAS.
- K. Bandyopadhyay, S. G. Liu, H. Liu and L. Echegoyen, Chem.–Eur. J., 2000, 6, 4385 CAS.
- P. W. Atkins, Physical Chemistry, PWN, Warsaw, 2001, pp. 827–829 Search PubMed.
- F. Mallamace, N. Micali, A. Romeo and L. M. Scolaro, Curr. Opin. Colloid Interface Sci., 2000, 5, 49 CrossRef CAS.
- B. Zhao, L. Duan, W. Liu, Y. Q. Zhao and B. S. Yang, Chin. J. Inorg. Chem., 2011, 27, 245 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra08099h |
| This journal is © The Royal Society of Chemistry 2014 |