Multiplicate sensitization of novel near-infrared luminescent linear copolymers based on Er, Nd and Yb-complexes†
Jianxin Luo*ab,
Chunyan Zhangab,
Changhong Lia,
Hanxiang Hua and
Bonian Hua
aKey Laboratory of green functional building materials, Department of Materials and Chemical Engineering, Hunan Institute of Technology, Hengyang, Hunan 421002, PR China. E-mail: luojianxin392@163.com; Tel: +86-0150-96045469
bInstitute of Organic Polymer Materials, Hunan Institute of Technology, Hengyang, Hunan 421002, PR China
First published on 23rd October 2014
Abstract
A series of novel near-infrared (NIR) luminescent linear copolymers, PCzLnQL2 (Cz = carbazole; Ln = Er, Nd, and Yb; Q = 8-hydroxyquinoline; L = 8-hydroxyquinoline, 5-[(carbazole-9-yl)methyl]-8-hydroxyquinoline and 2-thenoyltrifluoroacetone) covalently linked with Ln-complexes were synthesized and characterized. The obtained copolymers exhibit appropriate molecular weight, good solubility-processability and thermal stability for PLEDs application. The photophysical properties of the copolymers were studied by UV-vis absorption, steady state and transient fluorescence spectra. Monitoring the characteristic emission of the corresponding Ln3+, the copolymers show a broad excitation band extending from UV light to the region of visible light. Upon excitation with UV-vis light, the copolymers display high efficient near-infrared (NIR) luminescence of the corresponding Ln3+. Effects of N-vinylcarbazole segments and ligands on the NIR-luminescence of Ln3+ were investigated carefully. The results indicate that the NVK segments are not only used as light-harvesting groups, but also as a barrier to form a special microenvironment for the Ln-complex moieties. The three ligands can sensitize the three lanthanide ions, but the sensitized efficiency is not identical. Based on the observed luminescence phenomena and the reported energy transfer theories, the energy transfer mechanism for the NIR-luminescence of the copolymers was also investigated, which will provide a rule for designing perfect NIR-luminescence materials.
1. Introduction
In recent years, near-infrared (NIR) luminescent lanthanide ions such as erbium (Er), neodymium (Nd) and ytterbium (Yb), have attracted much attention as emissive materials in photonic devices,1 such as light emitting diodes (LEDs),2–4 active optical waveguides,5–9 lasers7–9 and bio-imaging probes.9–11 However, they have an intrinsically low molar absorption coefficient due to their forbidden characteristic of intra-4f transitions, which is difficult to generate efficient NIR-luminescence by direct excitation of these lanthanide ions.7 Generally, one solution to enhance NIR-luminescence of these lanthanide ions is to indirectly excite the lanthanide ions through an effective energy transfer from the triplet state of organic chromophores to the luminescent excited state of the lanthanide ions. The organic chromophores may be a ligand direct coordinated with lanthanide ions; or a sensitizer covalently attached on a simple ligand that coordinated with lanthanide ions. Recently, NIR-luminescence originating from sensitizer-functionalized ligand-based lanthanide complexes have been reported widely.12–19 Nevertheless, the inappropriate single sensitizer may lead to low absorption efficiency and energy transfer efficiency, and following lead to low NIR-luminescent efficiency. The co-excitation of multiplicate sensitizers may improve the NIR-luminescent efficiency of lanthanide ions.As is well known, polymers possess a number of advantages because of their low-cost, flexibility, good solution-processability and convenient control of various optical parameters such as refractive index, birefringence, and optical transparency bandwidth.20 Particularly, all kinds of functional groups can be covalently attached on the polymers linkages. As a consequent, plenty of functional polymers applied as luminescent materials have been designed and synthesized.20–26 For example, several red-luminescent copolymers containing [Eu(β-diketonate)] moieties and N-vinylcarbazole segments were synthesized by copolymerization of Eu-complexes monomers and N-vinylcarbazole.21–23 Intramolecular energy transfer from the carbazole groups to the europium complex moieties were confirmed in the photoluminescence process of the copolymers solution and film. Moreover, the copolymers were used as luminescent materials to prepare single layer high luminance polymeric light-emitting diodes (PLEDs). In addition, a series of bipolar Alq3-based copolymers containing Alq3 moieties and carbazole segments were obtained in our previously work, which showing high efficient energy transfer from the carbazole segments to Alq3 moieties.25 We also reported a NIR-luminescent linear copolymer based on tris(8-hydroxyquinoline)erbium, which has good solution-processability, thermal stability, and NIR-luminescent properties.27
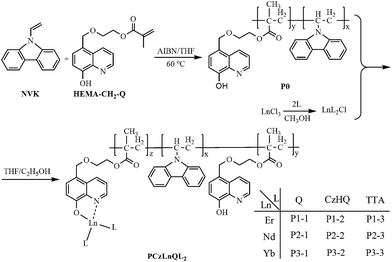
In this work, a series of NIR-luminescent linear copolymers containing Ln-complex (Ln = Er, Nd, and Yb) moieties and N-vinylcarbazole segments were designed and synthesized. A polymerizable 8-hydroxyquinoline derivate with methacrylate, 5-(2-methacryloytethyloxymethyl)-8-quinolinol (HEMA–CH2–Q), was copolymerized with N-vinylcarbazole (NVK) to obtain a polymeric ligand P0 firstly. Meanwhile, three different ligands (8-hydroxyquinoline, 5-[(carbazole-9-yl)methyl]-8-hydroxyquinoline and 2-thenoyltrifluoroacetone) were coordinated with three lanthanide ions to obtain nine Ln-complex precursors respectively. The nine Ln-complex precursors following coordinated with the polymeric ligand P0 to prepare nine linear copolymers containing Ln-complex (Ln = Er, Nd, and Yb) moieties and N-vinylcarbazole segments. The effects of the ligands and NVK segments on the NIR-luminescence of the Ln ions were investigated in detail. Moreover, the energy transfer mechanism in the NIR-luminescence of the copolymers were studied and proposed.
2. Experimental
2.1. Materials
8-Hydroxyquinoline (Q), triethylamine (NEt3), and all solvents were obtained commercially and used as received. 2-Thenoyltrifluoroacetone (TTA) was purchased from Aldrich Chemical Company. Ln2O3 (Ln = Er, Nd and Yb; 99.99%) was purchased from a Chinese company. N-vinylcarbazole (NVK) and azobisisobutyronitrile (AIBN) were purified and dried before use. 5-(2-Methacryloylethyloxymethyl)-8-quinolinol (HEMA–CH2–Q) and 5-[(carbazol-9-yl)methyl]-8-hydroxyquinoline (CzHQ) and tris(8-hydroxyquinoline) neodymium (NdQ3) was prepared according to the reported procedure.25,26 LnCl3 ethanol solution was obtained as described in ref. 15 The rare earth oxide (Ln2O3) was dissolved in concentrated hydrochloric acid (HCl), and the surplus HCl was removed by evaporation. The residue was dissolved with anhydrous ethanol. The concentration of the rare earth ion was measured by titration with a standard ethylenediaminetetraacetic acid (EDTA) aqueous solution.2.2. Synthesis of the copolymers
The synthesis route of the copolymers is shown in Scheme 1. Firstly, polymeric ligand (P0) was prepared according to the previous report.27 The mole ratio of HEMA–CH2–Q/NVK in feed is 1/50. Thereafter, nine Ln-complexes precursors (LnL2Cl·2H2O) were obtained according to the reference with slightly modification.16 About 10 mL ethanol solution of LnCl3 (concentration: about 1 mol L−1) was added to 20 mL methanol solution of ligands (concentration: 1 mol L−1) under stirring with the molar ratio of Ln3+/L being 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. Additionally, about 10 mL water was added. After stirring at 65 °C for 10 h, about 30 mL water was added to the mixture and more precipitates were obtained. The yellow precipitates were collected by filtration, washed with water and cold methanol for three times.
2. Additionally, about 10 mL water was added. After stirring at 65 °C for 10 h, about 30 mL water was added to the mixture and more precipitates were obtained. The yellow precipitates were collected by filtration, washed with water and cold methanol for three times.
Then, 5 mmol LnL2Cl·2H2O and a proper amount of P0 (the mole ratio of LnL2Cl·2H2O to the HQ in P0 is 5/1) were dissolved in 30 mL THF, and heated under reflux for one day. Meanwhile, appropriate NEt3 was added to adjust pH ≈ 7. Thereafter, the reaction mixture was cooled to room temperature and purified by several solution-precipitation cycles. The resulting solid was further purified by Soxhlet extraction with boiling methanol for two days and finally dried in a vacuum oven at 70 °C for 24 h.
2.3. Synthesis of the reference copolymers
Firstly, a polymeric ligand containing HEMA–CH2–Q and MMA (mole ratio in feed: HEMA–CH2–Q/MMA = 1/50) was prepared according to the reported procedure.26 Then, the polymeric ligand was coordinated with the Nd-complex precursor LnL2Cl·2H2O to obtain a copolymer (PMMA–NdQ3) containing NdQ3 moieties and MMA segments, as shown in Fig. 1S,† which was expected as a reference copolymer to investigate the effect of NVK segments on the NIR-luminescence of the titled copolymers.2.4. Characterization
The FT-IR spectra were carried out using a RFX-65A (Analects) Fourier Transform Infrared Spectrometer. Elemental analyses (C, H, and N) were performed with a Vario EL elemental analyzer, and lanthanide ion was analyzed by complexometric titration with EDTA. The molecular weight of the copolymer was determined by Waters 1515-2414 GPC gel permeation chromatography, using THF as an eluent and polystyrene as the standard. Differential scanning calorimetry (DSC) made on Pyris Diamond TA LAB system at a heating rate of 20 °C min−1 under nitrogen. Thermogravimetric analysis (TGA) was performed with Pyris 1 TGA instrument at a heating rate of 10 °C min−1 under nitrogen atmosphere. UV-vis absorption spectra of the polymer solutions (concentration: 0.01g L−1, solvent: THF) were determined on a Shimadz spectrophotometer. The polymer films were prepared under identical conditions by KW-4A Spin Coater. The thickness of polymer films was measured by an Alpha-step 500 surface profiler and is found to be about 100 nm. The excitation and emission spectra and the time-resolved measurements of the polymer films were recorded by an Edinburgh FLS 920 fluorescence spectrometer. The NIR luminescence lifetime of these polymers were measured at room temperature by using an excitation wavelength of 390 nm and monitored around the most intense emission line. The luminescent decay curve was fitted by double exponential functions. The emission lifetime of carbazole in these polymers were measured at room temperature by using an excitation wavelength of 305 nm and monitored around the most intense emission.3. Results and discussion
3.1. Synthesis and characterization
These linear copolymers, involving N-vinylcarbazole (NVK), isolated 8-hydrxoyquinoline (Q), ligands (Q, CzHQ and TTA) and lanthanide ions, were synthesized through the ligand exchange reaction according to the reported procedure,16 as shown in Scheme 1. All of the obtained copolymers exhibit good solubility in a wide range of organic solvents, such as toluene, THF, chloroform, DMF and DMSO. GPC measurements reveal that the weight average molecular weight (Mw) of the copolymers, as shown in Table 1, is around 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, which is higher than that of the polymeric ligand P0. Due to the dissolubility and appropriate molecular weight, the copolymers can be easily cast into uniform thin films with good mechanical flexibility.
000 g mol−1, which is higher than that of the polymeric ligand P0. Due to the dissolubility and appropriate molecular weight, the copolymers can be easily cast into uniform thin films with good mechanical flexibility.
| Copolymer | Contents (wt%) | Mole ratio of x/y/z | Mw (104 g mol−1) | PDI | Tg (°C) | Tda (°C) | Rwb (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| C | H | N | Ln | |||||||
| a The temperature at which 5% weight loss of the copolymer was reached from TGA under nitrogen atmosphere.b The weight percentage of the residual determined from TGA at 800 °C.c The data of P1-1 were obtained from our previous report.27 | ||||||||||
| P0 | 86.48 | 5.71 | 7.19 | 0 | 51/1/0 | 1.57 | 2.09 | 181 | 342 | 0 |
| P1-1c | 84.94 | 5.60 | 7.15 | 1.47 | 51/0.07/0.93 | 1.64 | 2.32 | 200 | 368 | 2.13 |
| P1-2 | 85.08 | 5.57 | 7.17 | 1.34 | 51/0.13/0.87 | 1.62 | 2.29 | 212 | 371 | 1.95 |
| P1-3 | 83.68 | 5.51 | 7.06 | 1.53 | 51/0.02/0.98 | 1.60 | 2.17 | 202 | 366 | 2.06 |
| P2-1 | 85.08 | 5.58 | 7.16 | 1.30 | 51/0.05/0.95 | 1.66 | 2.25 | 201 | 367 | 1.89 |
| P2-2 | 85.19 | 5.57 | 7.18 | 1.20 | 51/0.10/0.90 | 1.65 | 2.34 | 214 | 370 | 1.78 |
| P2-3 | 83.86 | 5.52 | 7.08 | 1.32 | 51/0.02/0.98 | 1.59 | 2.19 | 201 | 368 | 1.84 |
| P3-1 | 84.85 | 5.57 | 7.14 | 1.54 | 51/0.06/0.94 | 1.70 | 2.13 | 201 | 372 | 2.11 |
| P3-2 | 84.97 | 5.56 | 7.16 | 1.45 | 51/0.09/0.91 | 1.67 | 2.28 | 211 | 374 | 2.07 |
| P3-3 | 83.64 | 5.50 | 7.06 | 1.57 | 51/0.03/0.97 | 1.62 | 2.31 | 201 | 369 | 2.04 |
According to the contents of these elements (C, H, N, and Ln), the mole ratio of NVK/Q/LnQL2 (x/y/z) in the copolymers was calculated roughly and presented in Table 1. The results indicate that the titled copolymers have been synthesized successfully. Due to steric effect of the bulky and rigid LnL2Cl·2H2O, covalently linked 8-hydroxyquinoline in the polymer ligand P0 can not be coordinated with lanthanide ion completely, consequently, there are always some isolated 8-hydroxyquinoline groups in the copolymers. Moreover, one can found that large bulk CzHQ (P1-2, P2-2 and P3-2) leads to higher content of non-coordinating 8-hydroxyquinoline groups in the corresponding copolymers compared with other copolymers.
The FT-IR absorption spectra of the copolymers have been measured and exhibit similar absorption peaks, as shown in Fig. 2S.† Compared with the polymeric ligand, the copolymers have four new absorption peaks at 1264 cm−1, 802 cm−1, 525 cm−1 and 423 cm−1, assigned to the asymmetric stretching vibration of aryl ether, ring deformation, the stretching of O–Ln and N–Ln, respectively.28 Moreover, the intensity of stretching vibration of O–H (3430 cm−1) and C–O (1097 cm−1 and 1022 cm−1) increases in comparison with those of the polymeric ligand. All of the results suggest that the titled copolymers have been synthesized successfully.
3.2. Thermal properties
The thermal stabilities of the copolymers were studied in comparison with that of the polymeric ligand P0 and evaluated by means of differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) under nitrogen atmosphere. The obtained thermal properties data are listed in Table 1. The glass transition temperature (Tg) and the temperature of 5% weight loss (Td) for the copolymers are above 200 °C and 365 °C respectively, which increased about 20 °C in comparison with those of the polymer ligand. These results indicate that the copolymers have excellent thermal stability PLEDs application. The polymeric ligand has been decomposed completely at 800 °C, but about 2.00% of residual percentage weight at 800 °C assigned to Ln2O3 was observed for the copolymers.3.3. UV-vis absorption properties
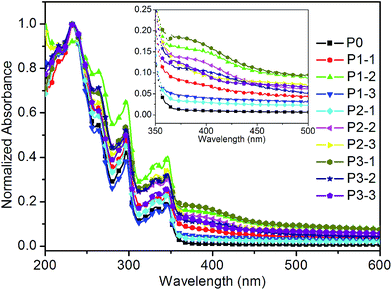
Normalized UV-vis absorption (Abs) spectra of the polymeric ligand and these copolymers in solution are shown in Fig. 1. All of the absorption spectra have five main absorption peaks of carbazole in the wavelength region of 200 nm to 350 nm: three strong absorption peaks around 238, 261 and 295 nm are attributable to the 1A → 1Ca, 1A → 1La and 1A → 1Ba transition of carbazole groups, respectively; two weaker peaks (330 and 345 nm) correspond to the 1A → 1Lb transition of carbazole groups.22 These results indicate that NVK segments are the main composition in both the polymeric ligand and the copolymers. Since the content of the LnQL2 moieties in these copolymers is rather low, the characteristic absorption band of the LnQL2 is rather weak. However, a new absorption band around 390 nm arised from metalloquinolate can be observed in the insert of Fig. 1, which further suggests that Ln-complex precursors are coordinated with polymeric ligand successfully.3.4. Photoluminescence (PL) properties
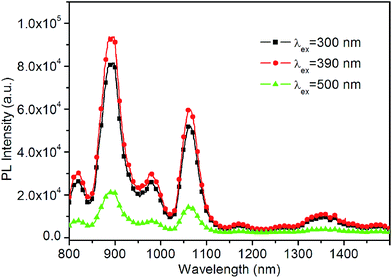
The photoluminescence spectra of the P1-series copolymers containing Er-complexes are shown in Fig. 2. In the excitation spectra of these copolymers, the broad bands extending to 500 nm are assigned to the absorption of metalloquinolate. Except for the LnQL2 moieties, the main components of these copolymers are identical (NVK segments). Therefore, the excitation spectra of these copolymers are similar. Upon excitation of metalloquinolate at 390 nm, the emission spectra of these P1-series copolymers were obtained. The emission bands cover large spectrum ranges, extended from 1450 to 1650 nm, which attributes to the transition from the first excited state to the ground state (4I13/2 → 4I15/2) of the Er3+. The full width at half-maximum (FWHM) of the 4I13/2 → 4I15/2 transition for P1-1 and P1-2 are 95 and 111 nm, respectively. In comparison with other Er-complex materials, the FWHM of the copolymers are quite broad, which is necessary for getting a wide gain bandwidth for optical amplification.8 | ||
| Fig. 2 PL excitation (Ex) and emission (Em) spectra of the P1-series copolymers containing Er-complexes in film (λem = 1525 nm, λex = 390 nm). | ||
Fig. 3 displays the PL excitation and emission spectra of the P2-series copolymers containing Nd-complexes. The excitation spectra were obtained by monitoring the main emission of Nd3+ at 1060 nm. A broad excitation band from UV light to visible light can be observed, which is assigned to the absorption of metalloquinolate, superimposed with excitation band (at 581 nm) originating from the characteristic absorption transition 4I9/2 → 2G17/2 of the Nd3+. It should be noted that the absorption transition at 581 nm of the Nd3+ is much weaker than that of the ligands, which indicate that the PL sensitization via exciting the ligands is much more efficient than directly exciting the absorption energy level of the Nd3+. The PL emission spectra of these P2-series copolymers were obtained upon excitation of metalloquinolate (λex = 390 nm), as shown in Fig. 3. The emission spectra of these copolymers consist of three main bands around 886 nm, 1060 nm, and 1340 nm, corresponding to the f–f transitions of 4F3/2 → 4I9/2, 4F3/2 → 4I11/2, and 4F3/2 → 4I13/2, respectively. In addition, there are two apparent emission peaks around 817 nm and 980 nm, which may be assigned to the f–f transitions of 4F5/2 → 4I9/2, and 4F5/2 → 4I11/2 respectively. Moreover, the strongest emission was observed around 886 nm, which is different with the previous reported.7,8 These phenomenons may be attributing to the function of the NVK segments.
 | ||
| Fig. 3 PL excitation (Ex) and emission (Em) spectra of the P2-series copolymers containing Nd-complexes in film (λem = 1060 nm, λex = 390 nm). | ||
The excitation spectra of the P3-series copolymers containing Yb-complexes in film (Fig. 4) were obtained by monitoring the characteristic emission of the Yb3+ at 980 nm. The excitation spectra are dominated by a broad band ranging from 300 to 550 nm, which should be assigned to the absorption of metalloquinolate. The emission spectra of the P3-series copolymers (Fig. 4) were obtained by direct excitation of metalloquinolate (λex = 390 nm). Except for the difference of emission intensity, the emission spectra of these copolymers are similar. In all of the curves, the prominent 980 nm emission band can be observed, which is assigned to the 2F5/2 → 2F7/2 transition of Yb3+. It should be noted that the emission band of Yb3+ is not a single sharp transition but an envelope of bands arising at lower energies. Similar splitting has been reported previously,7,8,15,16 which result from the crystal field splitting.29
 | ||
| Fig. 4 PL excitation (Ex) and emission (Em) spectra of the P3-series copolymers containing Yb-complexes in film (λem = 980 nm, λex = 390 nm). | ||
Monitoring the characteristic emission of the corresponding Ln3+, all of the copolymers show broad excitation band extended to the region of visible light. Excited with different wavelength (300–500 nm) of UV-vis light, the copolymer P2-1 displays similar emission peaks except for the difference of emission intensity, as shown in Fig. 5. Excited by 390 nm or 300 nm light, the copolymer P2-1 generates more intense NIR emission than that excited by 500 nm. The results indicate that the copolymers have broadband sensitized NIR-luminescence property, which provides an access to multicomposite in optical application, such as in medical diagnostic probe, laser, and optical amplifications.8
The lifetime measurement of the copolymers was investigated by using an excitation wavelength of 390 nm and monitored around the main emission of their corresponding emission spectra. The decay curve for P2-1 is single exponential, while the decay curves of other copolymers can be fitted by the double-exponential function. The values of the lifetime (τ1 and τ2) and the corresponding fractions (α1 and α1) are listed in Table 2.
| Copolymer | τ1/α1 (μs)/(%) | τ2/α2 (μs)/(%) | 〈τ〉 (μs) | φ (%) | kDA (105 s−1) | τq (ns) | τu (ns) | kEnT (107 s−1) |
|---|---|---|---|---|---|---|---|---|
| P1-1 | 12.57/35.2 | 2.23/64.8 | 5.87 | 0.042 | 1.70 | 7.6 | 13.4 | 5.70 |
| P1-2 | 14.90/13.7 | 1.97/86.3 | 3.74 | 0.027 | 2.67 | 7.3 | 13.4 | 6.24 |
| P1-3 | 1.54/84.9 | 12.06/15.1 | 3.11 | 0.022 | 3.22 | 7.1 | 13.4 | 6.62 |
| P2-1 | — | — | 1.46 | 0.584 | 6.85 | 5.9 | 13.4 | 9.49 |
| P2-2 | 1.16/87.5 | 15.05/12.5 | 2.90 | 1.160 | 3.45 | 4.2 | 13.4 | 16.35 |
| P2-3 | 1.13/83.0 | 12.5/17.0 | 3.06 | 1.224 | 3.27 | 3.8 | 13.4 | 18.85 |
| P3-1 | 15.69/48.1 | 6.53/51.9 | 10.94 | 0.547 | 0.91 | 4.7 | 13.4 | 13.81 |
| P3-2 | 3.19/77.8 | 11.09/22.2 | 4.94 | 0.247 | 2.02 | 5.9 | 13.4 | 9.49 |
| P3-3 | 1.34/86.1 | 13.58/13.9 | 3.04 | 0.152 | 3.29 | 6.4 | 13.4 | 8.16 |
According to the values of the lifetime and the corresponding fractions, the average PL lifetimes of the copolymers were calculated according to equation:  , and also presented in Table 2. The average PL lifetimes of the P1-series copolymers lay in the region of 3.11 to 5.87 μs, which is one order of magnitude higher than that of hybrid materials covalently linked with tris(8-hydroxyquinolinate)-erbium.8 In addition, the average PL lifetimes of the P2-series and P3-series copolymers are also higher than that of the corresponding Ln-complexes materials.8,15
, and also presented in Table 2. The average PL lifetimes of the P1-series copolymers lay in the region of 3.11 to 5.87 μs, which is one order of magnitude higher than that of hybrid materials covalently linked with tris(8-hydroxyquinolinate)-erbium.8 In addition, the average PL lifetimes of the P2-series and P3-series copolymers are also higher than that of the corresponding Ln-complexes materials.8,15
However, these lifetimes are much shorter than the intrinsic radiative lifetime of lanthanide ions excited state (τRAD), which is due to the non-radiative (NR) deactivation of NIR transition of lanthanide ions. Furthermore, the intrinsic radiative lifetime (τRAD) is far more than the non-radiative lifetime (τNR). Therefore, 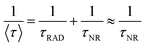 . According to ref. 30 and 31, the rate constants of non-radiative deactivation,
. According to ref. 30 and 31, the rate constants of non-radiative deactivation,  , were determined and listed in Table 2. By considering that the PL efficiency (φ) is determined by equation φ = 〈τ〉/τ0 (τ0 is the intrinsic radiative lifetime of the lanthanide ions excited state, the values of τ0 for Er3+, Nd3+ and Yb3+ are 14 ms, 0.25 ms and 2 ms). The PL efficiencies (NIR emission efficiency) of all the copolymers, listed in Table 2, are also higher than that of the corresponding Ln-complexes.32,33 According to the PL emission lifetime of carbazole in both polymeric ligand (τu) and copolymers (τq), as shown in Table 2, the energy transfer rate can be determined accord to equation kEnT = τq−1 − τu−1. The energy transfer rates are also listed in Table 2. The results indicate that the energy transfer rate determines the NIR emission efficiency to some extent and most of the energy transferred may lead to NIR emissions.
, were determined and listed in Table 2. By considering that the PL efficiency (φ) is determined by equation φ = 〈τ〉/τ0 (τ0 is the intrinsic radiative lifetime of the lanthanide ions excited state, the values of τ0 for Er3+, Nd3+ and Yb3+ are 14 ms, 0.25 ms and 2 ms). The PL efficiencies (NIR emission efficiency) of all the copolymers, listed in Table 2, are also higher than that of the corresponding Ln-complexes.32,33 According to the PL emission lifetime of carbazole in both polymeric ligand (τu) and copolymers (τq), as shown in Table 2, the energy transfer rate can be determined accord to equation kEnT = τq−1 − τu−1. The energy transfer rates are also listed in Table 2. The results indicate that the energy transfer rate determines the NIR emission efficiency to some extent and most of the energy transferred may lead to NIR emissions.
3.5. Effects of NVK segments on the NIR-luminescence of Ln3+
As discussed above, the average PL lifetimes and PL efficiencies of the copolymers are higher than that of the corresponding Ln-complexes reported previously. The results can be explained by the function of the NVK segments in the PL of the copolymers. In order to study the function of the NVK segments in the PL of the copolymers, both P0 and the copolymer P2-1 are excited with excitation wavelength of 305 nm, and the emission spectra of the copolymers in film are presented in Fig. 6. As can be seen, the characteristic emission of carbazole groups in P2-1 was quenched largely in comparison of that of P0. Moreover, the near-infrared luminescence intensity of P2-1 is higher than that of NdQ3 and the reference copolymer (PMMA–NdQ3), as shown in Fig. 7. All of the results suggest that the excitation energy of the carbazole in P2-1 can transfer to LnQL2 moieties effectively, and following enhanced the near-infrared luminescence of LnQL2 moieties.As mentioned above, the copolymers involve NVK segments, three different ligands and Ln ions. The Ln ions are used as luminescence center, which coordinated with ligands to form LnQL2 moieties. The mole ratios of NVK segments and LnQL2 moieties in the copolymers are about 51/1. It is mean that each LnQL2 moiety is surrounded with about 5l NVK segments. NVK segments encircle the LnQL2 moieties to form a special microenvironment. Due to the bulky and hydrophobic of the carbazole groups, water and solvent are prevented outside of the special microenvironment of the LnQL2 moieties to some extent. As a consequent, the nonradiative decay associated with the vibration of O–H or C–H bonds is restrained partly, and also following enhanced the NIR-luminescence of LnQL2 moieties.
Based on the two reasons discussed above, the average PL lifetimes and PL efficiencies of the copolymers are improved compared to the corresponding Ln-complexes. Therefore, we can come to the farther conclusion that the NVK segments are used as both light-harvesting group and barrier for enhancing the near-infrared luminescence of LnQL2 moieties.
3.6. Effects of ligands on the NIR-luminescence of the Ln3+
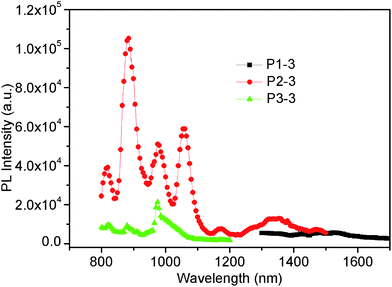
To study the effects of ligands on the NIR-luminescence of the Ln3+, the PL excitation and emission spectra of these copolymers containing different ligands were compared and presented in Fig. 2–4 respectively. Comparing the PL spectra of the P1-series copolymers containing Er-complex (Fig. 2), the intensity order is P1-1 > P1-2 > P1-3. Moreover, the PL efficiencies of the P1-series copolymers listed in Table 2 also show the same sequence. These results reveal that the sensitized efficiency of the ligands for Er3+ is: Q > CzHQ > TTA. In other words, 8-hydroxyquinoline is the most appropriate ligand for Er3+. For Nd3+, the PL intensity order is P2-2 > P2-3 > P2-1, as shown in Fig. 3, indicating CzHQ is the most appropriate ligand for Nd3+. Also, the PL spectra of P3-series copolymers containing Yb-complex were compared and depicted in Fig. 4. Both PL intensity and efficiency orders of the P3-series copolymers are P3-1 > P3-2 > P3-3, which also indicate that the sensitized efficiency of the ligands for Yb3+ is: Q > CzHQ > TTA.According to the discussion above, we know the sensitized order of different ligands for a special lanthanide ion. Thereinafter, the sensitized order of different lanthanide ions with a special ligand was also investigated according to Fig. 8–10. As can be seen from Fig. 8, we can found that the emission intensity order of the copolymers with 8-hydroxyquinoline ligand is P3-1 > P2-1 > P1-1. The result indicates that the sensitization of 8-hydroxyquinoline ligand for Yb3+ is the most effective, and for Er3+ is the worst. As for CzHQ, the emission intensity order of the Ln3+ is Nd (P2-2) > Yb (P3-2) > Er (P1-2), as shown in Fig. 9. This order reveal that the sensitized order of CzHQ for different lanthanide ions is Nd > Yb > Er. Similarly, the sensitized order of TTA for different lanthanide ions is also Nd > Yb > Er, as indicated in Fig. 10.
 | ||
| Fig. 8 PL emission (Em) spectra of the copolymers based on 8-hydroxyquinoline ligand in film (λex = 390 nm). | ||
3.7. Energy transfer mechanism
As is well known, energy transfer is based on the energy level difference between the excited triplet state of the ligand and the resonance energy level of the central rare earth ion. Too large or too small energy level differences decrease the efficiency of energy transfer.34 The suitable energy level difference for an efficient ligand-to-Ln3+ intramolecular energy transfer lies in the range of 500–2500 cm−1.34 There are four chromophores in the copolymers: carbazole (Cz), 8-hydroxyquinoline (Q), ligands (Q, CzHQ and TTA) and Ln3+. In order to further investigate the energy transfer mechanism, the excitation state energy levels of the chromophores (Cz, Q, TTA and Ln3+) were obtained from the previous reported ref. 27, 28, 34 and 35, and depicted in Scheme 2. Additionally, the excitation state energy levels of CzHQ were calculated by Gauss soft, and also depicted in Scheme 2.According to the energy level diagram, the energy level difference between the excited triplet state of the three ligands (Q, CzHQ and TTA) and the excited state of the Ln3+ is appropriated for energy transfer. It is mean that the energy can transfer from the excited triplet state of the three ligands to the high excited state of Ln3+, and following to the emission state of the Ln3+, which leads to NIR emission of Ln3+. However, part of energy has been consumed in the process of energy transfer from high excited state to the emission state of the Ln3+. Due to relative higher excited triplet state energy level, the energy transfer from the excited triplet state of TTA to the emission state of Ln3+ should go through more excited state than that of the other ligands. Therefore, the sensitized efficiency of TTA for Ln3+ is low than that of the other two ligands. In addition, one can found that the excited triplet state energy level of carbazole (∼25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1) is too high and the energy level difference with the emission state energy level of Ln3+ (Er3+: ∼7000 cm−1, Nd3+: ∼12
000 cm−1) is too high and the energy level difference with the emission state energy level of Ln3+ (Er3+: ∼7000 cm−1, Nd3+: ∼12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1, Yb3+: 11
000 cm−1, Yb3+: 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1) is too large (ΔE > 13
000 cm−1) is too large (ΔE > 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1). The energy level difference between the excited triplet state energy level of carbazole and the highest excited state energy level of Ln3+ is also above 6000 cm−1. Moreover, the distance between the carbazole and Ln3+ is long in the copolymers. Thus, energy transfer from carbazole to Ln3+ directly is not very efficient. However, the excited state energy of carbazole can transfer to the ligands and following to the Ln3+ as discussed in our previous report.27 Thus, no apparent characteristic emission of carbazole was observed in the PL spectra of the copolymer under different excitation wavelength.
000 cm−1). The energy level difference between the excited triplet state energy level of carbazole and the highest excited state energy level of Ln3+ is also above 6000 cm−1. Moreover, the distance between the carbazole and Ln3+ is long in the copolymers. Thus, energy transfer from carbazole to Ln3+ directly is not very efficient. However, the excited state energy of carbazole can transfer to the ligands and following to the Ln3+ as discussed in our previous report.27 Thus, no apparent characteristic emission of carbazole was observed in the PL spectra of the copolymer under different excitation wavelength.
4. Conclusions
A series of novel near-infrared (NIR) luminescent linear copolymers, involving N-vinylcarbazole (NVK), isolated 8-hydrxoyquinoline (Q), ligands (Q, CzHQ and TTA) and lanthanide ions, have been prepared and characterized. The copolymers exhibit appropriate molecular weight as well as good solubility-processability. The glass transition temperature (Tg) and the temperature of 5% weight loss (Td) for the copolymers are above 200 °C and 365 °C respectively, which will be advantageous for fabrication of optoelectronic device. The UV-vis absorption and photoluminescence properties of the copolymers were studied. The results indicate that the obtained copolymers show broadband sensitized and highly efficient near-infrared luminescence, which provide an access to multicomposite in optoelectronic applications, such as in optical amplifications, medical diagnostic, laser, and optics, etc. Effects of N-vinylcarbazole segments and ligands on the NIR-luminescence of Ln3+ were investigated. The NVK segments are not only used as light-harvesting group, but also as barrier to form a special microenvironment for the LnQL2 moieties. The three ligands can sensitize the three lanthanide ions, but the sensitized efficiency is different. Based on the observed luminescence phenomenons and the reported energy transfer theories, energy transfer mechanism for the NIR-luminescence of the copolymers was proposed, which will provide rule for designing perfect NIR-luminescence materials.Acknowledgements
This project was supported by the National Natural Science Foundation of China (Grant no. 21176061), Hunan Provincial Natural Science Foundation of China (Grant no. 13JJ4109), Scientific Research Fund of Hunan Provincial Education Department (Grant no. 13C203) and the Construct Program of the Key Discipline in Hunan province.Notes and references
- J.-C. G. Bünzli and S. V. Eliseeva, J. Rare Earths, 2010, 28, 824 CrossRef.
- L. H. Sloof, A. Polman, F. Caeialli, R. H. Friend, G. A. Hebbink, F. C. J. M. Veggel van and D. N. Reinhoudt, Appl. Phys. Lett., 2001, 78, 2122 CrossRef PubMed.
- Z. Q. Chen, F. Ding, Z. Q. Bian and C. H. Huang, Org. Electron., 2010, 11, 369 CrossRef CAS PubMed.
- Z. Li, J. Yu, L. Zhou, H. J. Zhang, R. P. Deng and Z. Y. Guo, Org. Electron., 2008, 9, 487 CrossRef CAS PubMed.
- P. C. Beeker, N. A. Olsson and J. R. Simpson, Erbium-doped fiber amplifiers: fundamentals and technology, AeademiePress, NewYork, 1999 Search PubMed.
- J.-C. G. Bünzli and C. Piguet, Chem. Soc. Rev., 2005, 34, 1048 RSC.
- X. M. Guo, H. D. Guo, L. S. Fu, L. D. Carlos, R. A. S. Ferreira, L. N. Sun, R. P. Deng and H. J. Zhang, J. Phys. Chem. C, 2009, 113, 12538 CAS.
- L. N. Sun, S. Dang, J. B. Yu, J. Feng, L. Y. Shi and H. J. Zhang, J. Phys. Chem. B, 2010, 114, 16393 CrossRef CAS PubMed.
- K. Kuriki, Y. Koike and Y. Okamoto, Chem. Rev., 2002, 102, 2347 CrossRef CAS PubMed.
- S. Faulker, S. J. A. Pope and B. P. Burton, Appl. Spectrosc. Rev., 2005, 40, 1 CrossRef PubMed.
- J. C. G. Bünzli, Chem. Lett., 2009, 38, 104 CrossRef.
- F. Pointillart, A. Bourdolle, T. Cauchy, O. Maury, Y. L. Gal, S. Golhen, O. Cador and L. Ouahab, Inorg. Chem., 2012, 51, 978 CrossRef CAS PubMed.
- H. B. Xu, J. Li, L. X. Shi and Z. N. Chen, Dalton Trans., 2011, 40, 5549 RSC.
- H. S. He, L. P. Si, Y. H. Zhong and M. Dubey, Chem. Commun., 2012, 48, 1886 RSC.
- S. Dang, J. B. Yu, X. F. Wang, Z. Y. Guo, L. N. Sun, R. P. Deng, J. Feng, W. Q. Fan and H. J. Zhang, J. Photochem. Photobiol., A, 2010, 214, 152 CrossRef CAS PubMed.
- L. N. Sun, H. J. Zhang, J. B. Yu, S. Y. Yu, C. Y. Peng, S. Dang, X. M. Guo and J. Feng, Langmuir, 2008, 24, 5500 CrossRef CAS PubMed.
- X. J. Zhu, W. K. Wong, W. Y. Wong and X. P. Yang, Eur. J. Inorg. Chem., 2011, 4651 CrossRef CAS.
- M. E. Gallina, C. Giansante, P. Ceroni, M. Venturi, J. Sakamoto and A. D. Schlüter, Eur. J. Inorg. Chem., 2011, 1479 CrossRef CAS.
- P. F. Yan, S. Chen, P. Chen, J. W. Zhang and G. M. Li, CrystEngComm, 2011, 13, 36 RSC.
- D. Liu and Z. G. Wang, Polymer, 2008, 49, 4960 CrossRef CAS PubMed.
- Q. D. Ling, Y. Song, J. Ding, C. Zhu, D. S. H. Chan, D. L. Kwong, E. T. Kang and K. G. Neoh, Adv. Mater., 2005, 17, 455 CrossRef CAS.
- Q. D. Ling, Q. J. Cai, E. T. Kang, K. G. Neoh, R. R. Zhu and W. Huang, J. Mater. Chem., 2004, 14, 2741 RSC.
- Z. G. Zhang, J. B. Yuan, H. J. Tang, H. Tang, L. N. Wang and K. L. Zhang, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 210 CrossRef CAS.
- H. Xu, R. Zhu, P. Zhao, L. H. Xie and W. Huang, Polymer, 2011, 52, 804 CrossRef CAS PubMed.
- J. X. Luo, C. L. Yang, J. Zheng, J. Y. Ma, L. Y. Liang and M. G. Lu, Eur. Polym. J., 2011, 47, 385 CrossRef CAS PubMed.
- Q. B. Mei, N. Y. Du and M. G. Lu, Eur. Polym. J., 2007, 43, 2380 CrossRef CAS PubMed.
- J. X. Luo, C. Y. Zhang, C. L. Yang and M. G. Lu, Synth. Met., 2012, 162, 431 CrossRef CAS PubMed.
- J. Thompson, R. I. R. Blyth, G. Gigli and R. Cingolani, Adv. Funct. Mater., 2004, 14, 979 CrossRef CAS.
- W. G. Perkins and G. A. Crosby, J. Chem. Phys., 1965, 42, 407 CrossRef CAS PubMed.
- A. Monguzzi, R. Tubino, F. Meinardi, A. O. Biroli, M. Pizzotti, F. Demartin, F. Quochi, F. Cordella and M. A. Loi, Chem. Mater., 2009, 21, 128 CrossRef CAS.
- A. Monguzzi, A. Milani, L. Lodi, M. I. Trioni, R. Tubino and C. Castiglioni, New J. Chem., 2009, 33, 1542 RSC.
- A. Nonat, D. Imbert, J. Pécaut, M. Giraud and M. Mazzanti, Inorg. Chem., 2009, 48, 4207 CrossRef CAS PubMed.
- N. M. Shavaleev, R. Scopelliti, F. Gumy and J. C. G. Bünzli, Inorg. Chem., 2008, 47, 9055 CrossRef CAS PubMed.
- X. M. Guo, H. D. Guo, L. S. Fu, H. J. Zhang, L. D. Carlos, R. P. Deng and J. B. Yu, J. Photochem. Photobiol., A, 2008, 200, 318 CrossRef CAS PubMed.
- M. D. Ward, Coord. Chem. Rev., 2007, 251, 1663 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra08093a |
| This journal is © The Royal Society of Chemistry 2014 |