Nanostructured self-cleaning lyocell fabrics with asymmetric wettability and moisture absorbency (part I)†
Seong-ok Kwona,
Tae-Jun Kob,
Eusun Yub,
Jooyoun Kimc,
Myoung-Woon Moon*b and
Chung Hee Park*a
aDepartment of Textiles, Merchandising, and Fashion Design, Seoul National University, Seoul, Korea. E-mail: junghee@snu.ac.kr
bInstitute of Multidisciplinary Convergence of Matter, Korea Institute of Science and Technology, Seoul, Korea. E-mail: mwmoon@kist.re.kr
cDepartment of Apparel, Textiles, and Interior Design, Kansas State University, Manhattan, KS, USA
First published on 29th August 2014
Abstract
A single-faced superhydrophobic lyocell fabric maintaining its inherent high moisture absorbing bulk property was produced by oxygen plasma-based nanostructuring and a subsequent coating with a low-surface-energy material. After 5 minutes of oxygen plasma etching, followed by 30 seconds of a plasma polymerized hexamethyldisiloxane coating, the treated surface of lyocell turned into a superhydrophobic surface with a static contact angle greater than 160° and a sliding angle less than 2°; however, the backside was hydrophilic, untreated lyocell fabric. As a result of oxygen plasma etching, dual hierarchical roughness was formed on the lyocell fabric as nano scale pillars or hairs were added onto the lyocell fabric surface with micro scale roughness. Extremely opposite wetting behavior was observed, when a water droplet was deposited on the face and backside of the plasma-treated lyocell fabric. A water droplet was immediately absorbed and spread out on the untreated backside, while it rolled off the treated surface, demonstrating a bouncing effect.
Introduction
Lyocell is known as a sustainable, biodegradable regenerated cellulose fiber1 with excellent mechanical properties2 and moisture absorption capability because of its unique fibril structure.3–5 However, because of the superhydrophilic and high water absorbing nature of lyocell fiber, the fabric can be easily contaminated by liquids, such as water, oil, beverages, or blood. Thus, it would add practicality if the lyocell fabric is modified to be water repellent, while the other side maintains its original wettability.The hydrophobicity of a material is governed by both the chemical composition of the material and the hierarchical geometric structure, which consists of micro and nano-scale roughness. When a water droplet contacts a rough surface, small-scale bumps trap air between them, reducing the contact area between the solid surface and the water. In this state, the surface is not fully wet and the water drop rolls off easily from the surface, carrying away any contaminant that is present.6 The fabrication of a superhydrophobic surface, defined as a surface exhibiting a static water contact angle (CA) greater than 150° and a water sliding angle or shedding angle (SA) less than 10°, has been inspired mostly by mimicking the existing structures in nature, such as lotus leaves, legs of water strider and insect wings.7
There have been numerous studies conducted in the fabrication of a superhydrophobic textile surface, including dip coating,8 sol–gel process,9 layer by layer assembly,10 electro spinning,11,12 spray coating,13 and plasma treatment.14,15 The most common technique used to create surface roughness, in order to reproduce the superhydrophobic nature, is to immerse the fabric in a solution containing nanoparticles. However, there is a potential health risk from exposure to the nanoparticles if they come off while wearing the fabric. Most superhydrophobic fabrics are hydrophobized after a roughening process, which might deteriorate the moisture-related comfort properties, such as sweat absorption and perspiration transmission.
To achieve both repellency and comfort properties, the fabrication of a single-faced superhydrophobic cotton fabric is achieved by the foam-coating of one face of the fabric with fluoropolymer emulsion.16 This method produces a fabric with asymmetric wetting properties, but the level of hydrophobicity achieved was not as high as that shown by the other method – plasma treatment16,17 – probably due to the lack of process sophistication.
Studies by Ko et al.17 demonstrated the effectiveness of plasma enhanced chemical vapour deposition (PECVD) process in fabricating the hydrophobic surface, where a plasma polymerized hexamethyldisiloxane (ppHMDSO) coating significantly lowered the surface energy of substrates. Furthermore, oxygen plasma etching with the subsequent PECVD of ppHMDSO engineered a superhydrophobic PET nonwoven surface, whose water CA was greater than 150°.18
In this study, plasma-based nanostructuring and chemical deposition was employed to produce superhydrophobic lyocell fabrics with extremely asymmetric wettability, and the resulting properties were characterized. For this objective, lyocell fabric was nanostructured on one face by etching with oxygen plasma with respect to the plasma treatment duration. With the subsequent deposition of a hydrophobic material, the superhydrophobic surface was fabricated. The chemistry of the nanostructured surface was analysed using X-ray photoelectron spectroscopy. The wettability was investigated by the measurement of water CA and SA at room temperature. The water absorption into the backside of the plasma treated lyocell fabric and asymmetric wetting behaviour was observed by a DSLR camera.
Experimental
Materials
228 g m−2 of 100% lyocell woven fabric, yarn count Ne 20 both warp and weft, fabric thickness of 0.38 mm was obtained from Shinjintex Co., Ltd. (Rep. Korea) and used as a substrate for plasma treatment. In regards to plasma reactor reagents, ultra-pure carrier grade of 99.99% O2 gas and HMDSO (Sigma Aldrich, USA) were used.Plasma processing
The plasma process for surface modification of the fabrics consisted of two steps: (1) reactive ion etching in the presence of oxygen to form a nano-hair structure on the fabric surface, and (2) subsequent thin film deposition of ppHMDSO. For the plasma process, specimens of 8 cm × 8 cm in size were placed on a stainless steel cathode plate, and the reactor was evacuated to a base pressure lower than 20 mTorr. Oxygen gas flow was initiated at a flow rate of 20 cm3 min−1. After the reactor reached a stable pressure of 20 mTorr, ion etching was started with a radio frequency glow discharge of oxygen gas at a bias voltage of −400 V.16 The duration of oxygen plasma etching was varied as 1, 3, 5, 10, and 20 minutes to yield various examples of surface roughness.After the etching process, deposition process of the thin film followed to enhance the hydrophobicity of the roughened structure by creating a thin layer of ppHMDSO coating with a surface energy of 24.4 mJ m−2.17 For the PECVD process, the reactor was evacuated to a base pressure of 1 mTorr, and HMDSO precursor gas was supplied at a flow rate of 10 cm3 min−1 with bias voltage of −400 V and a pressure of 10 mTorr for 30 seconds.
SEM and XPS analysis
Surface morphological changes after the plasma treatment were observed by field-emission scanning electron microscopy (FE-SEM, NanoSEM200, FEI, USA). The fabric specimen was sputter-coated with 10 nm thick Pt layer prior to the observation. X-ray photoelectron spectroscopy (XPS, ESCA System, PHI 5800, USA) was used to analyze the chemical composition and bonding of the untreated, oxygen-treated and O2 + HMDSO-coated specimens.Contact angle and shedding angle measurement
The static CA and SA of the water droplet were measured at room temperature using a contact angle goniometer, Attension® Theta Lite (Biolin Scientific, Sweden) equipped with a cradle, where a sliding angle of the substrate can be changed by 0.1° steps. For the measurements of static CA, a fabric specimen was mounted on a slide glass and placed on a sample stage. 4.06 ± 0.3 μl drops of deionized water were placed at different spots on the surface, and the CA was measured at five seconds after the placement of the water drops. For the SA measurements, the sliding angle at which a 12.5 ± 0.1 μl size water droplet rolls off at a distance of 2 cm or greater on the substrate was measured. The distance between the syringe tip and the fabric surface was fixed at 1 cm.19 All the measurements for CA and SA were conducted at 5 different spots for a specimen, and the average of 5 measurements was used.Results and discussion
Morphology of the nanostructured surfaces
As a synthetic cellulose fiber, the lyocell fiber surface is relatively smooth and uniform. Fig. 1(a) shows that there is almost no roughness existing on the fiber surface, unlike other cellulose fibers such as cotton or viscose rayon. However, immediately after 1 minute of oxygen plasma etching, nanoscale features were clearly formed on the surface of the lyocell fibers, as seen in Fig. 1(b).The SEM images in Fig. 1(b)–(f) reveal the formation of densely packed nano features because of oxygen plasma etching. The features grew longer and appeared to aggregate with increased plasma treatment after an etching time of 3 minutes. The phenomenon occurred because van der Waals' force between the nanohairs increased as the nano features grew with the increased etching time.20 It is interesting to note that the etching took place only on the fibers located in the front layer of the fabric, whereas the underlying fibers were partially etched at the area exposed to plasma gas, as seen in the lower magnified image of Fig. 1(d) and had a “shadow effect”. Moreover, it was confirmed that the pores remained intact after etching, implying uncompromised air permeability after the plasma etching and chemical deposition process.
As seen in Fig. 2(b), numerous nanometer scale features were formed on the top of the micro-size lyocell staple fibers, resulting in a dual hierarchical roughness. The dimensions of nano-features shown in SEM images were measured by the Image J program, as shown in Fig. 3(b).
 | ||
| Fig. 3 (a) Change of nano feature dimensions with increased plasma etching duration. (b) Benchmarks of measuring nano feature dimension by Image J. | ||
The average lyocell fiber diameter was measured to be 11.6 ± 1.2 μm, and the average size of nano features after 1 minute of oxygen plasma etching appeared to be 32.6 ± 3.2 nm wide and 67.0 ± 9.5 nm long. The length of the nano pillars substantially increased to 1112.4 ± 187.5 nm with increasing etching time, whereas the width decreased to 15.3 ± 3.5 nm. As shown in Fig. 3(a), the length of the nanoscale features proportionally increased with the etching duration at a rate of approximately 65 nm min−1.
As etching starts, the etch inhibitors (unetchable material like Fe, Al, Cr) from the reactor dome are deposited on the substrate surface (Fig. 4(b)). Where the etch inhibitors are not deposited, the cellulose lyocell is etched away by the reactive oxygen species, primarily O˙ and O*, forming water vapour, CO and CO2. When the reactive oxygen species perpendicularly collide with the specimen with high energy, they accelerate chemical and mechanical reactions on the horizontal surface. Whereas the reactions take place slowly on the vertical surfaces that are parallel to the oxygen ion movement. This anisotropic etching process appears to be the most important mechanism for the formation of rich nano features with high aspect ratios.21,22 This is in considerable agreement with the experimentally obtained data by Chen et al.,23 where the top and bottom diameters of the nano-pillar decrease with increasing oxygen plasma etching time.
Chemical modification of the surface
Surface atomic concentration and O/C ratio of the untreated lyocell specimen, oxygen plasma processed specimens, and oxygen plasma with HMDSO-treated specimens, are presented in Table 1.| Sample | Elements | O/C | |||
|---|---|---|---|---|---|
| Surface chemical composition (%) | |||||
| C1s | O1s | Si2p | Fe2p | ||
| a Note: O2 1–O2 10 are the oxygen treated specimens, where the numbers represent oxygen treated duration in minutes. The 5 minutes oxygen treated and HMDSO coated specimen is denoted as O2 5 + HMDSO. | |||||
| Untreated | 58.2 | 39.3 | 2.5 | 0 | 0.68 |
| O2 1 | 53.3 | 43 | 1.5 | 0.2 | 0.81 |
| O2 3 | 51.2 | 44.1 | 1.5 | 1.7 | 0.86 |
| O2 5 | 49.7 | 44 | 1.9 | 2.4 | 0.89 |
| O2 10 | 47.7 | 44.9 | 2.7 | 3.2 | 0.94 |
| O2 5 + HMDSO | 44.5 | 35.3 | 20.2 | 0 | 0.79 |
The untreated lyocell contained 58.2% of carbon and 39.3% of oxygen, which results in an O/C ratio of 0.68, which is similar to the previous finding by Peršin et al.24
The oxygen concentration gradually increased up to 44.9%, yielding an O/C ratio of 0.94 after 10 minutes of oxygen plasma etching. This indicates that the bombardment of reactive oxygen radicals enables the initiation of a chemical reaction, resulting in an alteration of chemical composition of the lyocell fabric. Although lyocell is a regenerated cellulose fiber and no natural impurities are expected, a small quantity of Si ranging from 1.5–2.7% was found in both untreated and plasma treated specimens. This might be because of the lubricants added to the fiber. However, the hydrophobic contaminations on the surface would have little influence on the wettability of the fibers because the wetting time of the untreated lyocell specimen was less than 0.03 seconds as seen in the ESI.†
Identification of bonding formation derived from the analysis of the C1s peak indicated that O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O bonds are newly formed on the oxygen etched lyocell specimen, as seen in Fig. 5. This is because the active O species (singlet 1O2, atomic O, anion-radical O−, and cation-radical O+) in the plasma reactor caused oxidation at C6, C1/C4 carbon, leading to the formation of –COOH on the fabric surface.25,26 After subsequent ppHMDSO deposition for 30 seconds, 20% of Si found on the treated surface confirmed the successful crosslinking of Si:Ox:Cy:Hz film on the roughened surface (Fig. 4(d)). A small quantity of Fe2p was also found after the etching process, and the concentration increased with increasing etching time, confirming the presence of an etching inhibitor in the oxygen etched specimens.
C–O bonds are newly formed on the oxygen etched lyocell specimen, as seen in Fig. 5. This is because the active O species (singlet 1O2, atomic O, anion-radical O−, and cation-radical O+) in the plasma reactor caused oxidation at C6, C1/C4 carbon, leading to the formation of –COOH on the fabric surface.25,26 After subsequent ppHMDSO deposition for 30 seconds, 20% of Si found on the treated surface confirmed the successful crosslinking of Si:Ox:Cy:Hz film on the roughened surface (Fig. 4(d)). A small quantity of Fe2p was also found after the etching process, and the concentration increased with increasing etching time, confirming the presence of an etching inhibitor in the oxygen etched specimens.
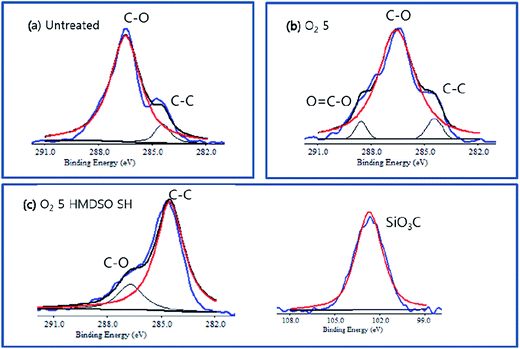 | ||
| Fig. 5 XPS spectra of (a) untreated (b) oxygen etched and (c) the surface of the O2 etched + HMDSO-coated. | ||
Effect of nanostructuring on the CA and the SA
The CA and the SA of the plasma-processed lyocell fabrics are shown in Fig. 6. Untreated lyocell instantly absorbed the water droplet, within 0.03 seconds, accordingly the CA was regarded to be 0°. With ppHMDSO coating alone, the CA remarkably increased to 147.6° with a SA at 9°. Only by lowering the surface energy of the fabric, which has micro roughness originating from the staple fibers, does the hydrophilic surface come near the superhydrophobic surface. This is in agreement with a previous study, which indicated that the cotton fabric can easily become hydrophobic after surface chemical modification, without adding roughness, because the staple fiber and woven textures themselves possess multi-scale microstructures.27 When nano-scale roughness was added to the structure by oxygen plasma processing followed by ppHMDSO coating, the CA was further increased to 151° and the SA was significantly lowered to 2.0°. With increasing oxygen etching, the CA gradually increased from 151° to 161° until 5 minutes etching time, and the lowest SA of 1.5° was achieved after 3 minutes of etching. This can be clearly explained by the nano-scale roughness drastically contributing to a lower SA close to 0° and thus performing self-cleaning function.The highest CA > 161° and the lowest SA < 1.5° in this study were observed from the treatment conditions of 5 minutes etching and 30 seconds ppHMDSO coating. Further etching durations did not significantly change the SA or static CA, regardless of the increased aspect ratio of the nanoscale feature. This could be attributed to the agglomerated nano hairs that would have increased the solid fraction of water and the hairs. It should be noted that a CA greater than 130° was maintained for 30 minutes, even though the CA was decreased to some extent, as the droplet size was reduced by evaporation (Fig. 6(b)). A water droplet pinned on the surface would have caused a decrease in CA because the evaporation does not occur at the pinned area of a droplet (Fig. 6(c)). A water droplet was not absorbed or wicked into the underlying hydrophilic fibers for the duration of 1 hour, confirming the stability of superhydrophobicity. It is interesting and meaningful to note that the other side of the plasma-processed lyocell showed 0° CA, as shown in the ESI,† regardless of the etching duration.
It is known that introducing surface roughness enhances the existing hydrophilicity or hydrophobicity of a solid surface based on the Wenzel's theory. With a low-surface-energy material coating on a fabric surface having micro scale roughness, the wetting properties of a flat hydrophobic surface can be further enhanced. This rule can be explained by the Cassie's law,28 mentioned in eqn (1).
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θc = fm(cos θc = fm(cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θm + 1) − 1 θm + 1) − 1
| (1) |
In a previous study,29 a complex wetting state on the dual-roughness structures was discussed with mathematical models based on energy variation analysis. By simple combination of the wetting states on different scale lengths, the superhydrophobic surfaces can be considered as a Cassie–Baxter regime in both the nano and micrometer scale. In this partial wetting state, a droplet is sitting only on the near-top surfaces of the roughness, at both nano (fn) and micro (fm) scales, leading to the apparent CA relationship as follows:
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θd = fmfn(cos θd = fmfn(cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θs + 1) − 1 θs + 1) − 1
| (2) |
 | (3) |
| O2 etching duration | Nano pillar size (nm) | fm | fn | Predicted CA | Measured CA | ||
|---|---|---|---|---|---|---|---|
| Diameter | Height | Space | |||||
| 1 | 32.6 | 67 | 25 | 0.34–0.5 | 0.3 | 151.2–156.3 | 151.3 ± 7.3 |
| 3 | 28.9 | 258.3 | 26 | 0.34–0.5 | 0.2 | 153.3–158.0 | 154.3 ± 5.9 |
| 5 | 27.1 | 319.4 | 28 | 0.34–0.5 | 0.2 | 155.0–159.5 | 161.2 ± 5.0 |
| 10 | 19.8 | 533.7 | 31 | 0.34–0.5 | 0.1 | 160.3–163.8 | 156.8 ± 7.0 |
| 20 | 15.3 | 1112.40 | 42 | 0.34–0.5 | 0.1 | 166.5–168.9 | 157.4 ± 5.0 |
Conclusion
An innovative superhydrophobic lyocell fabric having an extremely asymmetric wetting property was fabricated by oxygen plasma nanostructuring, and the subsequent deposition of hydrophobic ppHMDSO on the fabric surface. The opposite face of the fabric remained hydrophilic and moisture absorbent. After oxygen etching for longer than 1 minute, the plasma-processed lyocell surface showed nanostructures, which varied in morphology from pillar to hairy structures. The heights of nanostructures rapidly grew at a rate of 65 nm min−1, but the diameters were slightly reduced over time due to the anisotropic etching mechanism. With the subsequent hydrophobic coating, the surfaces were altered from their inherent nature of being hydrophilic into a superhydrophobic surface, exhibiting a CA over 150° and SA lower than 2°. The highest CA and lowest SA (161.2° and 1.5°, respectively) were achieved under the condition of 5 minutes of oxygen etching, followed by 30 seconds of ppHMDSO coating. The theoretically-predicted CA values based on the Cassie–Cassie equation30 of the nanostructured lyocell fabrics were comparable to the experimental data, until the oxygen etching time reached 10 minutes.As confirmed by XPS, the O2 plasma-processed lyocell showed a higher O/C ratio because oxygen can extract radicals from the surface of the lyocell, scissor the carbon chain, and/or introduce a variety of functional groups – especially a carbonyl group – in this study. While the O2 etched and HMDSO-coated surface confirmed the presence of a C:H:SiOx layer – a result of the plasma process – the chemical composition of the backside was comparable to that of the oxygen treated sample, confirming that the hydrophilicity was retained after the treatment.
The plasma-processed lyocell exhibited asymmetric wetting behaviour with superhydrophobic water repellency on the plasma-processed surface, and conversely, hydrophilic water absorbency on the backside of the treated surface. Achieving the asymmetric wetting properties on a fabric layer would be significant and relevant for applications that require water repellency and self-cleaning properties, and simultaneously not compromising the clothing comfort.
Further study on the moisture management properties, including absorption capability, wicking and drying behavior, would be meaningful and beneficial for the practical applications of this superhydrophobic textile.
Acknowledgements
This study was supported by the ‘BK21 Plus’ Project funded by the National Research Foundation of Korea in South Korea and by KIST internal project.References
- T. Rosenau, A. Potthast, H. Sixta and P. Kosma, Prog. Polym. Sci., 2001, 26, 1763–1837 CrossRef CAS.
- S. Peng, H. Shao and X. Hu, J. Appl. Polym. Sci., 2003, 90, 1941–1947 CrossRef CAS.
- M. Abu-Rous, K. Varga, T. Bechtold and K. S. Schuster, J. Appl. Polym. Sci., 2007, 106, 2083–2091 CrossRef CAS.
- H. Firgo, K. C. Schuster, F. Suchomel, J. Männer and T. Burrow, Lenzinger Ber., 2006, 85, 22–30 CAS.
- H. Firgo, F. Suchomel and T. Burrow, Lenzinger Ber., 2006, 85, 44–45 CAS.
- L. Feng, S. Li, Y. Li, H. Li, L. Zhang, J. Zhai, Y. Song, B. Liu, L. Jiang and D. Zhu, Adv. Mater., 2002, 14, 1857–1860 CrossRef CAS.
- Y. Zhao, Y. Tang, X. Wang and T. Lin, Appl. Surf. Sci., 2010, 256, 6736–6742 CrossRef CAS PubMed.
- M. H. Shim, J. Kim and C. H. Park, Text. Res. J., 2014, 84, 1268–1278 CrossRef PubMed.
- L. Xu, W. Zhuang, B. Xu and Z. Cai, Appl. Surf. Sci., 2011, 257, 5491–5498 CrossRef CAS PubMed.
- B. Leng, Z. Shao, G. de With and W. Ming, Langmuir, 2009, 25, 2456–2460 CrossRef CAS PubMed.
- D. W. Han and A. J. Steckl, Langmuir, 2009, 25, 9454–9462 CrossRef CAS PubMed.
- M. K. Sarkar, F. He and J. Fan, Thin Solid Films, 2010, 518, 5033–5039 CrossRef CAS PubMed.
- J. E. Mates, T. M. Schutzius, I. S. Bayer, J. Qin, D. E. Waldroup and C. M. Megaridis, Ind. Eng. Chem. Res., 2014, 53, 222–227 CrossRef CAS.
- K. Kale and S. Palaskar, Text. Res. J., 2010, 81, 608–620 CrossRef PubMed.
- J. Zhang, P. France, A. Radomyselskiy, S. Datta, J. Zhao and W. V. Ooij, J. Appl. Polym. Sci., 2003, 88, 1473–1481 CrossRef CAS.
- Y. Liu, J. H. Xin and C. H. Choi, Langmuir, 2012, 28, 17426–17434 CrossRef CAS PubMed.
- T. J. Ko, E. K. Her, B. Shin, H. Kim, K. Lee, B. K. Hong, S. H. Kim, K. H. Oh and M. W. Moon, Carbon, 2012, 50, 5085–5092 CrossRef CAS PubMed.
- B. Shin, K. Lee, M. Moon and H. Kim, Soft Matter, 2012, 8, 1817–1823 RSC.
- J. Zimmermann, S. Seeger and F. A. Reifler, Text. Res. J., 2009, 79, 1565–1570 CrossRef CAS PubMed.
- M. D. Volder and A. J. Hart, Angew. Chem., Int. Ed., 2013, 52, 2412–2425 CrossRef PubMed.
- K. Tsougeni, N. Vourdas, A. Tserepi, E. Gogolides and C. Cardinaud, Langmuir, 2009, 25, 11748–11759 CrossRef CAS PubMed.
- K. Ellinas, A. Tserepi and E. Gogolides, Langmuir, 2011, 27, 3960–3969 CrossRef CAS PubMed.
- X. L. Chen, Z. Liang, Z. Chen, W. Yang, T. Chen, C. Jin and B. Zhang, Chin. Phys. B, 2013, 22, 048101 CrossRef.
- Z. Peršin, A. Vesel, K. S. Kleinschek and M. Mozetič, Text. Res. J., 2012, 82, 2078–2089 CrossRef PubMed.
- K. Vaideki, S. Jayakumar and R. Rajendran, Plasma Chem. Plasma Process., 2009, 29, 515–534 CrossRef CAS PubMed.
- D. Caschera, Langmuir, 2013, 29, 2775–2783 CrossRef CAS PubMed.
- P. Roach, N. J. Shirtcliffe and M. I. Newton, Soft Matter, 2008, 4, 224–240 RSC.
- A. B. D. Cassie and S. Baxter, Trans. Faraday Soc., 1944, 40, 546–551 RSC.
- Y. Rahmawan, M. W. Moon, K. S. Kim, K. R. Lee and K. Y. Suh, Langmuir, 2010, 26, 484–491 CrossRef CAS PubMed.
- T. G. Cha, J. W. Yi, M. W. Moon, K. R. Lee and H. Y. Kim, Langmuir, 2010, 26, 8319–8326 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra08039d |
| This journal is © The Royal Society of Chemistry 2014 |




