Highly surface-roughened caterpillar-like Au/Ag nanotubes for sensitive and reproducible substrates for surface enhanced Raman spectroscopy†
Abstract
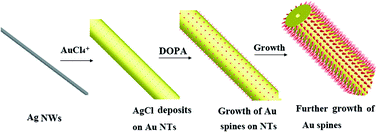
Caterpillar-like (CL) Au/Ag nanotubes (NTs) with high surface roughness were synthesized by a simple, rapid route using silver nanowires as sacrificial template and 3,4-dihydroxyphenylalanine (DOPA) as reducing and capping agent. Further experiments revealed that, by simply varying the ratios of the chlorauric acid and DOPA, the surface roughness of the CL Au/Ag NTs could be well controlled. The scanning electron microscope and transmission electron microscopy characterizations confirmed that the main NTs were polycrystalline, whereas the spines on their surface were single crystals. The hollow interior of the NTs was ascribed to the occurrence of the Kirkendall effect during the galvanic reaction between the metallic silver and AuCl4−. The formation of the spines results from the AgCl induced anisotropic growth of gold species. Because of their unique structures, such as a prickly surface and hollow internal tube, the as-prepared CL Au/Ag NTs exhibited a sensitive and reproducible performance in surface enhanced Raman scattering.

 Please wait while we load your content...
Please wait while we load your content...