Microwave-assisted derivatization for fast and efficient analysis of saccharides on disposable microchips†
Jinxiu Guo,
Shenghong Yang,
Xianglu Peng,
Fengyun Li,
Lei Zhou and
Qiaosheng Pu*
State Key Laboratory of Applied Organic Chemistry, Key Laboratory of Nonferrous Metals Chemistry and Resources Utilization of Gansu Province, Department of Chemistry, Lanzhou University, Lanzhou, Gansu 730000, China. E-mail: puqs@lzu.edu.cn; Fax: +86 931 891 2582; Tel: +86 931 8913813
First published on 23rd September 2014
Abstract
For rapid and low-cost determination of saccharides with microchip electrophoresis, a domestic microwave oven was used to perform the derivatization of saccharides with 8-aminonaphthalene-1,3,6-trisulfonate (ANTS) for laser-induced fluorescence detection with a 405 nm laser diode. The influence of the reaction conditions was systematically examined. Derivatization of xylose, glucose and lactose was fulfilled within 3 min under the optimized condition. With appropriate selection of the separation medium, these model analytes could be resolved within 30 s in borate solutions containing hydroxypropyl cellulose using disposable cyclic olefin copolymer microchips. Theoretical plate numbers of 1.0 × 106 m−1 and limits of detection of 0.15–0.23 μM were achieved. Peak areas were linear up to 1000 μM for all analytes. The applicability of the proposed method was verified by the determination of glucose in human urine and serum. Separation of oligosaccharides from konjac powder was also demonstrated. The domestic microwave oven, the cheap laser diode and disposable microchips used here make the method an economical way for rapid analysis of saccharides.
Introduction
Saccharides, the most abundant organic compounds found in the living world, are considered to be the energy and carbon sources of plants and animals. The glycan moieties of glycopeptides, glycoproteins, glycolipids and other glycoconjugates have been proven important in various biological events such as cell recognition, cell communication, cell proliferation, immune response and disease progression.1 Glycosylation is growing rapidly as most prominent post-translational modification occurring in many potential drugs.2 Glycomics,3 which deals with the comprehensive study of glycome, has drawn more and more attention recently. Quantitative analysis of saccharides is therefore important.However, the analysis of saccharides is still a challenge due to their structural complexity with microheterogeneity, and the lack of charged groups or chromophoric moieties.4 Currently, the preferred techniques of saccharide analysis are the chromatography and electrophoresis with different detection routes.4–6 Tedious chemical modifications are always needed in these techniques for certain purposes, such as improving thermal stability and volatility for gas-phase analyses, attaching fluorophores for optical detection and introducing charge for mass spectral analyses.6 Fast sample preparation, efficient separation and sensitive detection are hardly achievable concurrently, the development of rapid, efficient and sensitive methods for analysis of saccharides is still necessary.
As a newly emerging analytical technique, microchip electrophoresis (MCE) has been successfully used in the analysis of DNA fragments,7,8 amino acids,9 proteins,10,11 neurotransmitters,12 saccharides13,14 and other biological molecules with its outstanding advantages, such as high speed, low sample/reagent consumption and easy automation. MCE has shown capability of miniaturization and high sensitivity when laser-induced fluorescence detection (LIF) and electrochemical detection are used. Baba's group has proved that MCE-LIF was suitable for rapid separation of saccharides.14,15 Recently, we have developed economical electrophoresis devices including disposable cyclic olefin copolymer (COC) microchips,16 power supplies and LIF detectors that assembled with cheap optical and electronic parts.17 These home-made MCE devices are portable due to the relatively small dimensions (30.0 cm × 18.0 cm × 31.2 cm). They have been successfully employed for the rapid analysis of samples with complicated matrices.16–19 It should be possible to develop economical ways of saccharides analysis with these devices.
Derivatization is indispensable for introduction of fluorophores when MCE-LIF is used for saccharides. Some aromatic primary amines have been used as fluorescent tags based on reductive amination, such as 2-aminopyridine (AP),20 5-aminonaphthalene-2-sulfonic acid (5-ANSA),21 8-aminopyrene-1,3,6-trisulfate (APTS),14,15 2-aminoacrydone (AMAC)22 and 8-aminonaphthalene-1,3,6-trisulfonate (ANTS).23 ANTS was first demonstrated by Jackson24 for use in polyacrylamide-gel electrophoresis. The optimum excitation wavelength of ANTS is around 356 nm,25 but it can be readily excited with widely available low-cost diode lasers of 405 nm which are mainly used in DVD laser heads. The use of 405 nm laser diodes can ensure the low-cost nature of the apparatus. However, the derivatization time needed for ANTS is quite long,23,26 which significantly compromise the speed of MCE separation. Therefore, it is vital to develop a rapid and effective derivatization protocol for saccharides that matches the high-speed analysis of MCE.
The application of microwave in chemical reactions has been widely recognized including synthetic,27 environmental28 and analytical chemistry. Microwave-assisted sample extraction29 and digestion30 are typical examples in analytical area. We have previously demonstrated a rapid analysis method for histidine, 1-methylhistidine and 3-methylhistidine in human urine by using microwave-accelerated derivatization.31 Momenbeik and Khorasani32 reported a protocol for the pre-column derivatization of sugar samples with p-nitroaniline within 5 min under microwave irradiation before reversed-phase high-performance liquid chromatographic analysis with UV detection. However, microwave-assisted derivatization of saccharides before fluorescence detection was rarely seen.
In the present work, we incorporated microwave-assisted derivatization and MCE-LIF to realize rapid and efficient analysis of saccharides labeled with ANTS. Effects of experimental conditions were systematically investigated with xylose, glucose and lactose as the model analytes. The applicability of the proposed method was evaluated through the determination of glucose in human urine and serum samples. Its capability for analysis of oligosaccharides was evaluated by the separation of extracts of konjac powder, which is a kind of food supplement that was believed to be beneficial to control glycemia, lipids, and systolic blood pressure.33
Experimental
Apparatus
A laboratory-made LIF detector with a 405 nm laser diode (Sanyo, 20 mW), a dichroic mirror (430 nm, Shenyang HB Optical Technology Co., Ltd., Shenyang, China), a microscope objective (20×, Beijing 7-Star Optical Instruments Co., Ltd., Beijing, China), a long-pass filter (520 nm, Shenyang HB Optical Technology Co., Ltd.) and a photomultiplier tube (CR105, Beijing Hamamatsu Photon Techniques Co., Ltd.), which arranged in an epifluorescence configuration described previously17 was used for the detection. A three-way solenoid valve (WTB-3R-N4F, 12 VDC, 200 kPa, Takasago Electric Co., Ltd., Suzhou, China) and a programmable high voltage supply assembled with a high voltage module (DW-P602, Dongwen High-Voltage Power Supply, Tianjin, China) were controlled by an NI-6009 multifunctional card (National Instruments, Austin, TX) through a program written in LabVIEW (National Instruments) to automate injection and separation. The data acquisition and display of electropherograms were fulfilled by the same program.A domestic microwave oven (P70D20P-TD(W0), 700 W, Galanz, Guangzhou, China) was used for the derivatization reaction. To avoid damage to the magnetron caused by low load, continuous pumping of water was maintained through a PTFE tubing (2.0 mm i.d.) that was introduced into the microwave chamber through the vent holes (Fig. 1). The length of the tubing inside the chamber was approximately 30 cm, which contains 0.94 mL of water. Fluorescence spectra of ANTS and its sugar derivatives were recorded on a fluorescence spectrophotometer (F97Pro, Lengguang Technology Co., Ltd., Shanghai, China).
 | ||
| Fig. 1 Schematic illustration of the chamber of the microwave oven and the position of the ‘cooling coil’. | ||
Reagents and materials
Glucose, xylose, α-lactose monohydrate standards were purchased from Xi'an Zhoudingguo Chemical Reagent Factory (Xi'an, China). ANTS was obtained from Aladdin Chemistry Co., Ltd. (Shanghai, China). NaBH3CN was a product of Shanghai Jiachen Chemical Industry Co., Ltd. (Shanghai, China). Dimethyl sulfoxide (DMSO) was obtained from Lianlong Bohua Pharmaceutical Chemical Co., Ltd. (Tianjin, China). Hydroxypropyl cellulose (HPC) was from Bio Basic Inc. (Shanghai, China). Borax was product of Tianjin Guangfu Fine Chemical Research Institute (Tianjin, China). Glacial acetic acid and sodium hydroxide were purchased from the Tianjin Guangfu Science and Technology Development Co., Ltd. (Tianjin, China). Hydrochloric acid was from Beijing Chemical Factory (Beijing, China). Konjac powder was a product of Langao Zhushan Food industry Co., Ltd. (Ankang, China). Neutral β-mannanase was provided by Beijing Bosar Biotechnology Co., Ltd. (Beijing, China). All reagents except konjac powder and neutral β-mannanase were of analytical grade and used without further purification. Distilled water was used in all experiments.Stock standard solutions of xylose, glucose and lactose with concentrations of 0.1 M were prepared in distilled water and stored in a refrigerator (4 °C). The running buffers for MCE were prepared by mixing given amounts of hydroxypropyl cellulose to sodium tetraborate with appropriate concentrations. The pH of the running buffer was adjusted using 1.0 M hydrochloric acid or 1.0 M sodium hydroxide. All solutions were filtered through 0.45 μm membranes (Shanghai Xinya Purification Materials Factory, Shanghai, China) before use.
Sample preparation
Human urine samples were collected from 3 healthy males (labelled as Samples 1–3) and a man with diabetes (Sample 4). The urine samples were centrifuged at 4000 rpm for 10 min and stored at 4 °C. The blood was collected from the same volunteer at the same time into a vacuum tube without anticoagulant (Boda Medical Device Co., Ltd., Zibo, China). Serum was separated from the whole blood by centrifugation at 3900 rpm for 10 min at room temperature and stored at −20 °C. All samples were filtered through 0.45 μm membranes before derivatization.Glucomanno-oligosaccharides were extracted with the following procedure: 1.00 g konjac powder was slowly added into 100 mL distilled water and then mixed with 0.49 g neutral β-mannanase to start the reaction in a water bath at 41 °C. The pH of the mixture was adjusted to 7.0. After 3.4 h of incubation, the mixture was boiled for 15 min and centrifuged at 3900 rpm for 10 min.34 The supernatant was freeze-dried (DZF-6020, Shanghai Yiheng Technology Co., Ltd., Shanghai, China) to get glucomanno-oligosaccharides.
Derivatization with ANTS
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17, v/v) and 10 μL of freshly prepared 1 M NaBH3CN solution in DMSO were added sequentially into a 0.5 mL microcentrifuge vial. After vortex mixing, the microcentrifuge vial was capped with a small hole (0.3 mm i.d.) punched. The vial was placed in the microwave oven for 3 min. The pumping of water through PTFE tubing was started and a flow rate of 15 mL min−1 was used for all experiments. After cooling to ambient temperature, the samples were diluted with running buffer if necessary for MCE-LIF analysis.
17, v/v) and 10 μL of freshly prepared 1 M NaBH3CN solution in DMSO were added sequentially into a 0.5 mL microcentrifuge vial. After vortex mixing, the microcentrifuge vial was capped with a small hole (0.3 mm i.d.) punched. The vial was placed in the microwave oven for 3 min. The pumping of water through PTFE tubing was started and a flow rate of 15 mL min−1 was used for all experiments. After cooling to ambient temperature, the samples were diluted with running buffer if necessary for MCE-LIF analysis.Microchip electrophoresis
The detailed procedures for COC microchip fabrication16 and microchip electrophoresis18,19 were described previously. A negative pressure pinched sample injection17,18 was used for the separation. Briefly, the analytes in the sample reservoir were driven to the cross of the microchip for 1–3 s through vacuum switched by a 3-way solenoid valve. The high voltage (2000 V) was immediately applied over the buffer and buffer waste reservoirs to inject a narrow zone of sample into the separation channel and realize the electrophoretic separation. The fluorescence signal was collected by the LIF detector focused on the downstream of the separation channel. The effective separation length from cross to detection point was 3.0 cm.Results and discussion
Rapid separation of saccharides
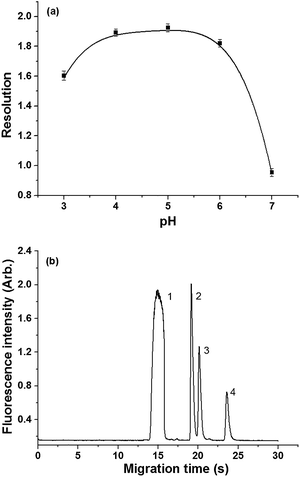
Because of saccharides' indispensable role in biological process and their importance as main components of various foods, rapid analysis of these compounds is necessary. MCE is one of the potential candidates due to its speed and capability for multi-component analysis. There is a tradeoff between separation length and performance. We found that the isomers of disaccharides could be separated on COC microchip with 8.0 cm separation length. HPC was added in the borate solutions as a viscosity regulator and surface modifier to suppress EOF and avoid tedious liquid level balancing among four reservoirs on the microchip.18 Because the relative bigger channel sizes (cross section area > 4000 μm2) of the microchips we used, the increased viscosity of the buffer did not impose any difficulty in microchannel filling and rinsing. Meanwhile, the bigger channel sizes are also beneficial to reduce the chance of microchannel clogging by air-borne particles, which helps improve the robustness of the system. To verify the performance of the system suitable for rapid analysis, xylose, glucose and lactose were selected as the model analytes. Influence of pH, concentration of borate as well as the concentration of HPC was firstly investigated.The effect of buffer acidity on saccharides separation was studied in a pH range of 3.0 to 8.0, while concentration of borate was maintained at 80 mM and HPC concentration was fixed at 1.0% (m/v). The results suggested that all analytes migrated to the anode and pH had profound effect on the resolution. Xylose and glucose could not fully resolved when pH > 7.0. At pH 4.0–6.0, all these compounds and ANTS were well separated. Hence, pH 5.0 was used for the following experiments based on the compromise of resolution and theoretical plate numbers (Fig. 2a and b).
The effect of borate concentration was examined in the range of 20–100 mM with 1.0% HPC at pH 5.0. The resolution between xylose and glucose was dependent on the borate concentration and reached the highest at 80 mM (Fig. S1a†). The migration time of all derivatives of saccharides decreased with increase of the borate concentration, most probably due to the further suppression of EOF toward the cathode.18 The electrophoretic behavior became unstable and tiny bubbles appeared in the separation channel when the concentration of borate increased to 100 mM. Therefore, 80 mM borate solution was selected for the further experiments.
The influence of HPC concentration on separation was studied from 0.5% to 3.0% (w/v) when borate solution was maintained at 80 mM and pH was adjusted to 5.0. With a small amount of HPC present in the running buffer, satisfactory resolution of three analytes could be achieved. When the concentration of HPC increased from 0.5% to 3.0%, migration time could increase by 79.8% for all analytes. The change of resolution between xylose and glucose was shown in Fig. S1b.† Based on the resolution, 1.0% HPC was selected.
When 80 mM of borate containing 1.0% HPC at pH 5.0 was used, xylose, glucose and lactose could be baseline separated within 30 s, which was pretty match the requirement of rapid and economical separation of saccharides. However, the derivatization needed at least one hour if the conventional procedure was adopted,35 which is approximately 100 times longer than separation. Exploring the efficient derivatization of saccharides that was compatible with the established MCE separation is critical for this work.
Microwave-assisted derivatization with ANTS
In order to reduce the total cost of the analysis, we used ANTS as the derivatization reagent to match the cheaper 405 nm diode laser, which is widely utilized in the DVD laser heads. The fluorescence intensity of ANTS-labeled glucose excited at 405 nm was 23.3% of that at its optimum excitation wavelength, 356 nm (Fig. S2†). However, because of easily achievable high power (can be 200 mW or higher) of 405 nm lasers and the excellent performance of LIF system, this offset of the excitation wavelength did not give rise to apparent detrimental effect on the performance.Traditional derivatization with ANTS or APTS takes much longer time than that for microchip electrophoretic separation. A domestic microwave oven was employed to perform the derivatization. As shown in Scheme S1,† the formation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond between a saccharide and ANTS is somewhat reversible. Further reduction of C
N bond between a saccharide and ANTS is somewhat reversible. Further reduction of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond to C–N leads to a more stable product. Microwave could improve both steps of the reaction due to the polar groups involved.36 To get the optimum condition, various factors were systematically investigated, including the amounts of reagents (ANTS and NaBH3CN), reaction time, and the location of the reaction vial in the microwave chamber.
N bond to C–N leads to a more stable product. Microwave could improve both steps of the reaction due to the polar groups involved.36 To get the optimum condition, various factors were systematically investigated, including the amounts of reagents (ANTS and NaBH3CN), reaction time, and the location of the reaction vial in the microwave chamber.
To run a domestic microwave oven without load may damage the oven. To avoid this type of damage to the magnetron caused by small load, a PTFE ‘cooling coil’ that was filled with flowing water was used. The volume of water in the PTFE tubing was only 0.94 mL, but it was sufficient to avoid the damage of the microwave oven even without any other load in the chamber.
Because the distribution of microwave inside the chamber is not uniform, the best location of the reaction vial should be defined. To find the best location, we put 27 vials (with exactly same reaction mixture) evenly distributed in 3 layers, 9 points in each layer (as shown in Fig. 3), and checked the derivatization performance in each vial. The peak areas of xylose obtained from the mixtures in every vial were plotted in Fig. 3. The best was the center of the bottom layer, which is the center of the rotary tray of the oven.
 | ||
| Fig. 3 Contours of peak area of the ANTS-derivatized xylose placed at 27 positions (circled in white) in the microwave chamber of the oven. Derivatization conditions: 0.2 M ANTS, reaction under microwave irradiation of 700 W for 3 min. X-, Y- and Z-directions were shown by axis in Fig. 1. Other conditions are same as in Fig. 2b. | ||
ANTS was commonly used for saccharides labeling via acid catalyzed reductive amination. ANTS provides both fluorescent tag and negative charge from three sulfonic groups, which enables neutral sugar molecules to migrate under the action of electric field.24,35 Polar solvents, especially water, can facilitate the absorption of microwave36 and are therefore favorable to the reaction. As shown in Fig. 4, the fluorescence intensities of ANTS labeled analytes increased evidently with the concentration of ANTS from 0.05 to 0.125 M and plateaued at higher concentrations. Therefore 0.125 M ANTS was used for the derivatization, which corresponds to 25 μL of 0.2 M ANTS added to the mixture. It was found that the concentration of acetic acid used to dissolve the ANTS affected the fluorescence intensity, too. We studied its influence in a concentration range of 0–25% (v/v). The results are shown in Fig. S3.† The maximum fluorescence intensities were achieved when 15% acetic acid was used in ANTS solution. Lower concentration of acetic acid gave rise to the apparent decrease of the signal, because acetic acid is a catalyst of the formation of the Schiff base,35 while too high concentration of acetic acid might protonate –NH2 of ANTS and lead to decrease of the signal. The effect of NaBH3CN was also examined, 0.25 M NaBH3CN gave the optimum results, as shown in Fig. 5.
 | ||
| Fig. 4 Effect of the concentration of ANTS. Conditions are same as in Fig. 3 except the microcentrifuge vial containing sample was placed at the center of the rotary tray. | ||
 | ||
| Fig. 5 Effect of the amount of NaBH3CN. Conditions are same as in Fig. 4. | ||
The output power of the domestic microwave oven can normally be adjusted by the ‘firepower’, but this adjustment is actually realized through switching the magnetron on and off intermittently.37 For the derivatization, we need microwave to be applied constantly, so 100% fire power (700 W) was used in all of our experiments. The fluorescence of all analytes increased with the increase of the time of microwave treatment. To evaluate the efficiency of the derivatization, the samples obtained with microwave was compared with that derivatized in an 80 °C boiling bath for 2.5 h, which was used in previous works.23 The experimental results indicated that 3 min was enough to reach the consequence of traditional derivatization, so 3 min was used for the next steps. The peak heights of xylose, glucose and lactose obtained with microwave were 126.1%, 97.7%, and 77.0% of that with water bath, respectively. The difference in the derivatization efficiencies between normal heating and microwave-assisted derivatization might be related to the molecular sizes that affect the motion and reorientation of the molecules under electromagnetic fields provided by the microwave.
Analytical performance
Linearity, limits of detection (LODs) and precision were evaluated (Table 1). When the peak areas (Y) were plotted versus concentrations of analytes (X), they were linear up to 1000 μM for all three analytes and the correlation coefficients were above 0.997. The reproducibility of the method was evaluated based on the migration time and peak area for all analytes. The relative standard deviations (RSDs) of migration time and peak area were not more than 1.9% and 3.1%, respectively. LODs (S/N = 3) were in a range of 0.15–0.23 μM, which were sufficient for most of practical applications. Although these LOD values are not better than that obtained with APTS (1.98 × 10−2 μM (ref. 38)), it still has plenty of space for the improvement by using higher laser power.| Sugars | Regression equation | LOD (μM) | LOQ (μM) | Linear range (μM) | RSD (%) | |||
|---|---|---|---|---|---|---|---|---|
| Slope (×10−4) | Intercept (×10−3) | Correl. coeff. | Migration time (n = 5) | Peak area (n = 5) | ||||
| Xylose | 8.51 | 4.29 | 0.9971 | 0.23 | 0.76 | 0.8–1000 | 0.88 | 2.7 |
| Glucose | 4.54 | 7.88 | 0.9995 | 0.16 | 0.55 | 0.6–1000 | 1.9 | 3.1 |
| Xylose | 4.42 | 2.69 | 0.9990 | 0.15 | 0.49 | 0.5–1000 | 0.40 | 2.9 |
Application and method validation
 | ||
| Fig. 6 Electropherograms of (a) human urine sample (Samples 1) (A), and one spiked with 1 mM of saccharides (B); and (b) human serum sample (A), and one spiked with 8 mM of saccharides (B). Conditions and peak identification are same as in Fig. 2b. The urine and serum samples were diluted by factors of 10 and 100, respectively. | ||
| Analyte | Content (mM) | Added (mM) | Totally found (mM) | Recovery (%) | Within-day RSD (%) (n = 5) | Between-day RSDb (%) (n = 3) |
|---|---|---|---|---|---|---|
| a Represents not detected.b Represents results detected in three days. | ||||||
| Xylose | n.d.a | 1.00 | 1.10 | 109.8 | 0.5 | 1.6 |
| 2.00 | 2.12 | 106.2 | 1.8 | 3.4 | ||
| 3.00 | 2.95 | 98.3 | 0.7 | 1.5 | ||
| Glucose | 0.62 | 1.00 | 1.64 | 102.5 | 1.3 | 2.9 |
| 2.00 | 2.64 | 101.3 | 2.8 | 3.8 | ||
| 3.00 | 3.82 | 106.7 | 1.7 | 2.6 | ||
| Lactose | n.d. | 1.00 | 1.05 | 105.3 | 0.9 | 0.6 |
| 2.00 | 1.95 | 97.6 | 2.4 | 2.1 | ||
| 3.00 | 3.34 | 111.4 | 1.4 | 1.9 | ||
For the method validation, three addition levels, 1.00, 2.00 and 3.00 mM sugar standards were spiked into urine samples, 5.00, 8.00 and 10.00 mM were spiked into serum samples to check the standard recoveries. These spiked saccharides in both types of real samples were determined successfully (Tables 2, 3 and S1†). The recoveries for all three addition levels were in a range of 91.0–118.3%. There was no significant difference in RSDs of within-day and between-day values and the maximum was below 5%.
| Analyte | Content (mM) | Added (mM) | Totally found (mM) | Recovery (%) | Within-day RSD (%) (n = 5) | Between-day RSD (%) (n = 3) |
|---|---|---|---|---|---|---|
| Xylose | n.d. | 5.00 | 5.60 | 112.1 | 3.1 | 3.7 |
| 8.00 | 7.42 | 92.8 | 1.8 | 2.9 | ||
| 10.00 | 10.24 | 102.4 | 0.6 | 4.7 | ||
| Glucose | 6.44 | 5.00 | 12.36 | 118.3 | 3.7 | 4.3 |
| 8.00 | 14.94 | 106.3 | 3.3 | 2.1 | ||
| 10.00 | 17.23 | 107.8 | 2.9 | 2.7 | ||
| Lactose | n.d. | 5.00 | 4.81 | 96.1 | 4.7 | 4.0 |
| 8.00 | 7.81 | 97.6 | 1.9 | 0.9 | ||
| 10.00 | 9.66 | 96.6 | 3.1 | 1.5 |
After microwave-assisted derivatization with ANTS, GMOs were separated on COC disposable microchips. Parameters including borate concentration (20–120 mM), HPC concentration (0.5–2.5%, m/v) and buffer pH (5.0–10.0) of running buffer were screened for improving the separation efficiency. The results suggested that the concentration of borate and HPC could significantly influence the migration time of GMOs, similar to that for xylose etc. Effect of acidity on the separation of oligosaccharides was negligible in a pH range of 5.0–10.0. The electropherogram of GMOs from konjac powder at pH 8.0 were shown in Fig. 7. Under this condition, at least seven components in GMO could be well resolved.
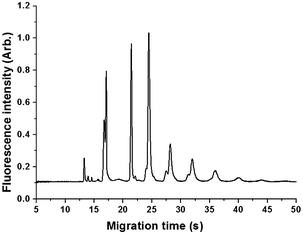 | ||
| Fig. 7 Electropherogram of glucomanno-oligosaccharides (GMOs) from konjac powder. Conditions are same as in Fig. 2b, except buffer pH was 8.0. | ||
Conclusions
The combination of microwave-assisted derivatization and MCE-LIF provides an ideal way for fast, economical, and efficient analysis of saccharides. Its applicability was verified through the determination of saccharides in human urine and serum with standard addition recoveries of 91.0–118.3%. The feasibility for the analysis of oligosaccharides was proved with extracts from konjac powder. The significance of the present work is the efficient analysis realized through the integration of domestic microwave oven based derivatization and the use of low-cost reagents and economical microchip electrophoresis platform based on widely available and cheap components. In addition, the LIF detectors and other components can be greatly miniaturized as shown in works reported previously.43,44 Miniaturized microwave is also available.45 It is therefore possible to make much smaller integrated devices that are more suitable for on-site analysis. The successful use of these low-cost components and reagents may significantly facilitate the analysis outside of professional laboratories and broaden the application area of microchip electrophoresis, especially in resource-limited areas.Acknowledgements
The authors would like to acknowledge financial support from the National Natural Science Foundation of China (no. 21175062, 21105040), the Specialized Research Fund for the Doctoral Program of Higher Education (20110211120010). F.Y. Li acknowledges the support from the Fundamental Research Funds for the Central Universities (lzujbky-2013-89).Notes and references
- C. R. Bertozzi and L. L. Kiessling, Science, 2001, 291, 2357–2364 CrossRef CAS PubMed.
- M. Dalziel, M. Crispin, C. N. Scanlan, N. Zitzmann and R. A. Dwek, Science, 2014, 343, 1235681 CrossRef PubMed.
- R. Raman, S. Raguram, G. Venkataraman, J. C. Paulson and R. Sasisekharan, Nat. Methods, 2005, 2, 817–824 CrossRef CAS PubMed.
- H. J. An, A. H. Franz and C. B. Lebrilla, J. Chromatogr. A, 2003, 1004, 121–129 CrossRef CAS PubMed.
- Y. C. Lee, J. Chromatogr. A, 1996, 720, 137–149 CrossRef CAS.
- D. J. Harvey, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2011, 879, 1196–1225 CrossRef CAS PubMed.
- A. T. Woolley, D. Hadley, P. Landre, A. J. deMello, R. A. Mathies and M. A. Northrup, Anal. Chem., 1996, 68, 4081–4086 CrossRef CAS PubMed.
- J. C. Albrecht, A. Kotani, J. S. Lin, S. A. Soper and A. E. Barron, Electrophoresis, 2013, 34, 590–597 CrossRef CAS PubMed.
- X.-T. Li, D. Xiao, X.-M. Ou, C. McCullm and Y.-M. Liu, J. Chromatogr. A, 2013, 1318, 251–256 CrossRef CAS PubMed.
- H. Shadpour and S. A. Soper, Anal. Chem., 2006, 78, 3519–3527 CrossRef CAS PubMed.
- S.-Y. Wang, S. K. Njoroge, K. Battle, C. Zhang, B. C. Hollins, S. A. Soper and J. Feng, Lab Chip, 2012, 12, 3362–3369 RSC.
- C. A. Croushore and J. V. Sweedler, Lab Chip, 2013, 13, 1666–1676 RSC.
- A. A. Kazarian, E. F. Hilder and M. C. Breadmore, Analyst, 2010, 135, 1970–1978 RSC.
- F.-Q. Dang, K. Kakehi, K. Nakajima, Y. Shinohara, M. Ishikawa, N. Kaji, M. Tokeshi and Y. Baba, J. Chromatogr. A, 2006, 1109, 138–143 CrossRef CAS PubMed.
- F.-Q. Dang, L.-H. Zhang, M. Jabasini, N. Kaji and Y. Baba, Anal. Chem., 2003, 75, 2433–2439 CrossRef CAS PubMed.
- Q. Wang, Y. Zhang, H. Ding, J. Wu, L.-L. Wang, L. Zhou and Q.-S. Pu, J. Chromatogr. A, 2011, 1218, 9422–9427 CrossRef CAS PubMed.
- L.-L. Wang, J. Wu, Q. Wang, C.-H. He, L. Zhou, J. Wang and Q.-S. Pu, J. Agric. Food Chem., 2012, 60, 1613–1618 CrossRef CAS PubMed.
- R.-N. Li, L.-L. Wang, X.-T. Gao, G.-F. Du, H.-L. Zhai, X.-Y. Wang, G.-S. Guo and Q.-S. Pu, J. Hazard. Mater., 2013, 248–249, 268–275 CrossRef CAS PubMed.
- X. Wei, X.-T. Gao, L. Zhao, X.-L. Peng, L. Zhou, J. Wang and Q.-S. Pu, J. Chromatogr. A, 2013, 1281, 148–154 CrossRef CAS PubMed.
- Y. Takegawa, K. Deguchi, S. Ito, S. Yoshioka, H. Nakagawa and S.-I. Nishimura, Anal. Chem., 2005, 77, 2097–2106 CrossRef CAS PubMed.
- Z.-X. Wei and M.-X. Wan, J. Appl. Polym. Sci., 2003, 87, 1297–1301 CrossRef CAS.
- E. Maeda, M. Kataoka, M. Hino, K. Kajimoto, N. Kaji, M. Tokeshi, J. Kido, Y. Shinohara and Y. Baba, Electrophoresis, 2007, 28, 2927–2933 CrossRef CAS PubMed.
- A. Klockow, R. Amado, H. M. Widmer and A. Paulus, J. Chromatogr. A, 1995, 716, 241–257 CrossRef CAS PubMed.
- P. Jackson, Biochem. J., 1990, 270, 705–713 CrossRef CAS PubMed.
- M. Iijima and Y. Nagasaki, J. Chromatogr. A, 2006, 44, 1457–1469 CAS.
- R. A. Evangelista, M. S. Liu and F. T. A. Chen, Anal. Chem., 1995, 67, 2239–2245 CrossRef CAS.
- N. Rajesh, R. Sarma and D. Prajapati, RSC Adv., 2014, 4, 7834–7837 RSC.
- K. Srogi, Anal. Lett., 2006, 39, 1261–1288 CrossRef CAS.
- A. A. Rahim, S. Nofrizal and B. Saad, Food Chem., 2014, 147, 262–268 CrossRef CAS PubMed.
- H. Matusiewicz, Analyst, 2009, 134, 1490–1497 RSC.
- L. Zhou, N. Yan, H.-G. Zhang, X.-M. Zhou, Q.-S. Pu and Z.-D. Hu, Talanta, 2010, 82, 72–77 CrossRef CAS PubMed.
- F. Momenbeik and J. H. Khorasani, Anal. Bioanal. Chem., 2006, 384, 844–850 CrossRef CAS PubMed.
- B. Li, J. Xia, Y. Wang and B.-J. Xie, J. Agric. Food Chem., 2005, 53, 7404–7407 CrossRef CAS PubMed.
- J.-F. Chen, D.-S. Liu, B. Shi, H. Wang, Y.-Q. Cheng and W.-J. Zhang, Carbohydr. Polym., 2013, 93, 81–88 CrossRef CAS PubMed.
- M. Stefansson and M. Novotny, Anal. Chem., 1994, 66, 1134–1140 CrossRef CAS PubMed.
- A. de la Hoz, A. Diaz-Ortiz and A. Moreno, Chem. Soc. Rev., 2005, 34, 164–178 RSC.
- A. A. Salema and F. N. Ani, Bioresour. Technol., 2011, 102, 3388–3395 CrossRef CAS PubMed.
- F.-Q. Dang, L.-H. Zhang, H. Hagiwara, Y. Mishina and Y. Baba, Electrophoresis, 2003, 24, 714–721 CrossRef CAS PubMed.
- C. Chen, Q.-J. Xie, D.-W. Yang, H.-L. Xiao, Y.-C. Fu, Y.-M. Tan and S.-Z. Yao, RSC Adv., 2013, 3, 4473–4491 RSC.
- A. M. Demchuk, L. B. Morgenstern, D. W. Krieger, T. L. Chi, W. Hu, T. H. Wein, R. J. Hardy, J. C. Grotta and A. M. Buchan, Stroke, 1999, 30, 34–39 CrossRef CAS PubMed.
- J. Charlwood, H. Birrell, E. S. P. Bouvier, J. Langridge and P. Camilleri, Anal. Chem., 2000, 72, 1469–1474 CrossRef CAS PubMed.
- C. M. Starr, R. I. Masada, C. Hague, E. Skop and J. C. Klock, J. Chromatogr. A, 1996, 720, 295–321 CrossRef CAS PubMed.
- B. Yao, H.-H. Yang, Q.-L. Liang, G.-A. Luo, L.-D. Wang, K.-N. Ren, Y.-D. Gao, Y.-M. Wang and Y. Qiu, Anal. Chem., 2006, 78, 5845–5850 CrossRef CAS PubMed.
- K.-N. Ren, Q.-L. Liang, X. Mu, G.-A. Luo and Y.-M. Wang, Lab Chip, 2009, 9, 733–736 RSC.
- S. Perino, E. Petitcolas, M. de la Guardia and F. Chemat, J. Chromatogr. A, 2013, 1315, 200–203 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1–S3, Scheme S1. See DOI: 10.1039/c4ra07934e |
| This journal is © The Royal Society of Chemistry 2014 |