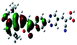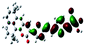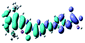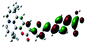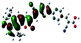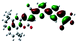Exploring photophysical properties of metal-free coumarin sensitizers: an efficient strategy to improve the performance of dye-sensitized solar cells†
Jinghui Wanga,
Ming Liab,
Dan Qia,
Wei Shena,
Rongxing He*ab and
Sheng Hsien Linc
aSchool of Chemistry and Chemical Engineering, Southwest University, Chongqing 400715, China. E-mail: herx@swu.edu.cn
bEducation Ministry Key Laboratory on Luminescence and Real-Time Analysis, Southwest University, Chongqing 400715, China
cDepartment of Applied Chemistry, Institute of Molecular Science and Center for Interdisciplinary Molecular Science, National Chiao-Tung University, Hsinchu 300, Taiwan
First published on 16th October 2014
Abstract
An efficient strategy was provided by adopting different numbers of electron-deficient units (pyrimidyl and quinolyl) into parent coumarin sensitizers to obtain excellent absorption in the short-wavelength region (B2 band), which eventually improves the performance of DSSCs. Density functional theory calculations were performed on both free dyes and dye–TiO2 complexes. As expected, introducing a single electron-deficient unit results in a positive influence on the power conversion efficiency (η) of DSSCs because of the larger short-circuit current density (Jsc is proportional to optical absorption (φLHE), charge separation, dye regeneration (φreg) and electron injection (φinject)) and the higher open circuit voltage (Voc). The introduction of more pyrimidine facilitates charge separation and favors effective electron injection, whereas the second quinoline displays the opposite effect. The results give guidance to design promising candidates for future DSSCs applications.
1. Introduction
Dye-sensitized solar cells (DSSCs) have received immense attention due to high power conversion efficiency (η) and low cost as compared to conventional silicon-based solar cells.1–3 So far Ru complexes are one of the most promising dyes for DSSCs with η > 11%, but the usage of rare and expensive ruthenium severely limits their application.2 Therefore, metal-free organic dyes (facile molecular design and high molar extinction coefficient) are now being proposed as an alternative to Ru-based sensitizers because they are cheaper, more abundant and less toxic.4–6Coumarin 343 (C343) and its derivatives2,7,8 are one of the most promising families of organic dyes because they undergo efficient and fast electron injection successfully used in DSSCs, but their performance is limited by narrow photoresponse range in the whole visible region, unwelcome π-stacked aggregation and undesirable charge recombination.9,10 For these reasons, the η of DSSCs using coumarin sensitizers is much lower than that based on Ru and Zn complexes.11 Therefore, Hara et al.12 expanded the π-conjugation by inserting more vinylene units (–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–) or π-conjugated rings13,14 (such as thiophene, benzene, furan or pyrrole) into the π-spacers and Rociosa et al.15 introduced –NH2 group into skeleton of coumarin to solve these problems. Despite these two strategies are helpful to broaden the absorption bands of coumarin sensitizers in long-wavelength region (Q band), the relatively weak and narrow absorption bands in short-wavelength region still severely attenuate the light capture capability of sensitizers and further restrict the efficiency of DSSCs.2,16 How to put forward appropriate countermeasures to improve the absorption properties (including the absorption range and light harvesting efficiency) in the whole visible region has become a topic much worthy of discussion. In this work, thus, different electron-deficient units were incorporated into π-spacers of dyes to improve the absorption intensity and broaden the absorption range of coumarin sensitizers in short-wavelength region. Experimentally, Wong et al.17 testified that the triphenylamine-based sensitizers bearing a pyrimidine unit display enhanced spectral responses in the red portion of the solar spectrum and exhibit high η. Subsequently, He et al.18 theoretically explored how the electron-deficient pyrimidine affected the performance of the porphyrin sensitizers, and clarified that the electron-deficient units would lead to redshift absorption and efficient charge separation. Most recently, He et al.19 further investigated the role of varied-length spacers in electron transfer, especially the influence of π-spacer modified by electron-deficient units on electron transfer. They found that electron injection driving force of model compounds gradually decreases as the increase of π-spacer length, whereas the introduction of electron-deficient pyrimidinyl breaks the dependence of π-bridge length. With all the information, we believe that the adoption of electron-deficient units into the π-spacer is a feasible strategy to improve η of DSSCs. On the basis of the theoretical interpretation together with the experimental results, two questions are worthy of considering. Firstly, it is found that the performance of DSSCs is improved when electron-deficient unit is adopted in π-bridge of metal organic dyes. So we are very curious to know whether the metal-free coumarin sensitizers that introduce electron-deficient units can also improve the light-harvesting efficiency and further raise the performance of DSSCs. Secondly, how do the different electron-deficient units and their amount influence absorption spectra, intrinsic characters of electron transition and different electron transfer processes of free sensitizers? It is not sufficient to assess the performance of DSSCs with the related characters of free dyes. Thus, the characters of dye–TiO2 complexes should be probed to understand the electron transfer process from the dyes to TiO2.8,10
CH–) or π-conjugated rings13,14 (such as thiophene, benzene, furan or pyrrole) into the π-spacers and Rociosa et al.15 introduced –NH2 group into skeleton of coumarin to solve these problems. Despite these two strategies are helpful to broaden the absorption bands of coumarin sensitizers in long-wavelength region (Q band), the relatively weak and narrow absorption bands in short-wavelength region still severely attenuate the light capture capability of sensitizers and further restrict the efficiency of DSSCs.2,16 How to put forward appropriate countermeasures to improve the absorption properties (including the absorption range and light harvesting efficiency) in the whole visible region has become a topic much worthy of discussion. In this work, thus, different electron-deficient units were incorporated into π-spacers of dyes to improve the absorption intensity and broaden the absorption range of coumarin sensitizers in short-wavelength region. Experimentally, Wong et al.17 testified that the triphenylamine-based sensitizers bearing a pyrimidine unit display enhanced spectral responses in the red portion of the solar spectrum and exhibit high η. Subsequently, He et al.18 theoretically explored how the electron-deficient pyrimidine affected the performance of the porphyrin sensitizers, and clarified that the electron-deficient units would lead to redshift absorption and efficient charge separation. Most recently, He et al.19 further investigated the role of varied-length spacers in electron transfer, especially the influence of π-spacer modified by electron-deficient units on electron transfer. They found that electron injection driving force of model compounds gradually decreases as the increase of π-spacer length, whereas the introduction of electron-deficient pyrimidinyl breaks the dependence of π-bridge length. With all the information, we believe that the adoption of electron-deficient units into the π-spacer is a feasible strategy to improve η of DSSCs. On the basis of the theoretical interpretation together with the experimental results, two questions are worthy of considering. Firstly, it is found that the performance of DSSCs is improved when electron-deficient unit is adopted in π-bridge of metal organic dyes. So we are very curious to know whether the metal-free coumarin sensitizers that introduce electron-deficient units can also improve the light-harvesting efficiency and further raise the performance of DSSCs. Secondly, how do the different electron-deficient units and their amount influence absorption spectra, intrinsic characters of electron transition and different electron transfer processes of free sensitizers? It is not sufficient to assess the performance of DSSCs with the related characters of free dyes. Thus, the characters of dye–TiO2 complexes should be probed to understand the electron transfer process from the dyes to TiO2.8,10
Herein, four model coumarin-derived chromophores (NKX-2311,10,12 NKX-2677,20–22 NKX-2700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23 and NKX-2883
23 and NKX-2883![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13,24 were investigated to confirm the significant role of π-spacer in promoting the η of DSSCs (see ESI† for detailed discussion). Then, different numbers of electron-deficient units (pyrimidine17 and quinoline25) were adopted into NKX-2677, NKX-2700 and NKX-2883 to discuss the two questions mentioned above. The structures of all investigated dyes were illustrated in Fig. 1. To further rationalize the strategy presented in this work, eight dye–TiO2 complexes were designed to investigate their geometrical and electronic structures, absorption spectra, electron injection efficiency.8 We believe that the present effort to address the requirements of highly efficient DSSCs is a helpful exploration.
13,24 were investigated to confirm the significant role of π-spacer in promoting the η of DSSCs (see ESI† for detailed discussion). Then, different numbers of electron-deficient units (pyrimidine17 and quinoline25) were adopted into NKX-2677, NKX-2700 and NKX-2883 to discuss the two questions mentioned above. The structures of all investigated dyes were illustrated in Fig. 1. To further rationalize the strategy presented in this work, eight dye–TiO2 complexes were designed to investigate their geometrical and electronic structures, absorption spectra, electron injection efficiency.8 We believe that the present effort to address the requirements of highly efficient DSSCs is a helpful exploration.
The stimulated absorption spectra of NKX-2677,22 NKX-2700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23 and NKX-2883
23 and NKX-2883![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 reproduce the experimental results very well for the first time, indicating that the present theoretical methods are reliable. Compared with the three parent coumarin dyes, the new designed sensitizers have more outstanding performance in optical absorption intensity and range. Generally, the strong spectral absorption does not always facilitate effectual charge separation, which means the performance of sensitizers is not only relied on the extrinsic spectral absorption intensity but also on the intrinsic character of electron movement upon photoexcitation.18 To assess the efficiency of charge separation, we apply a novel approach proposed by Le Bahers26,27 to quantify the charge transfer (CT) distance (L), the overlap (Ω) between the zones of density depletion and increment and the amount of transferred charge (Δe). The three parameters are evaluated only from the ground state (GS) and excited state (ES) total electronic densities. The central idea of this method is to compute the barycenters of the regions of increased/decreased of electronic densities. The results show that thorough charge-separation between the donor and the acceptor of sensitizers, and valid electron injection and regeneration can be gained by introducing electron-deficient units. Although the coumarin derivatives trigger intensive interest due to their application in DSSCs, as far as we know, theoretical studies on their dye–TiO2 complexes still remain rather limited. Moreover, the electron injection mechanism will be influenced strongly by the electronic coupling (V) between dye molecule and semiconductor conduction band.2,15 Thus, it is necessary to give a deeper insight into the performance of dye–TiO2 complexes, aiming to make sure the charge transfer and dye–TiO2 interaction occurring at the semiconductor interface. In order to demonstrate how the electron-deficient modified π-bridge improve the performance of the DSSCs, comprehensive investigation, including electronic structures, absorption spectra and electron transfer for isolated coumarin dyes and dye–TiO2 complexes, will be presented in the present work.
24 reproduce the experimental results very well for the first time, indicating that the present theoretical methods are reliable. Compared with the three parent coumarin dyes, the new designed sensitizers have more outstanding performance in optical absorption intensity and range. Generally, the strong spectral absorption does not always facilitate effectual charge separation, which means the performance of sensitizers is not only relied on the extrinsic spectral absorption intensity but also on the intrinsic character of electron movement upon photoexcitation.18 To assess the efficiency of charge separation, we apply a novel approach proposed by Le Bahers26,27 to quantify the charge transfer (CT) distance (L), the overlap (Ω) between the zones of density depletion and increment and the amount of transferred charge (Δe). The three parameters are evaluated only from the ground state (GS) and excited state (ES) total electronic densities. The central idea of this method is to compute the barycenters of the regions of increased/decreased of electronic densities. The results show that thorough charge-separation between the donor and the acceptor of sensitizers, and valid electron injection and regeneration can be gained by introducing electron-deficient units. Although the coumarin derivatives trigger intensive interest due to their application in DSSCs, as far as we know, theoretical studies on their dye–TiO2 complexes still remain rather limited. Moreover, the electron injection mechanism will be influenced strongly by the electronic coupling (V) between dye molecule and semiconductor conduction band.2,15 Thus, it is necessary to give a deeper insight into the performance of dye–TiO2 complexes, aiming to make sure the charge transfer and dye–TiO2 interaction occurring at the semiconductor interface. In order to demonstrate how the electron-deficient modified π-bridge improve the performance of the DSSCs, comprehensive investigation, including electronic structures, absorption spectra and electron transfer for isolated coumarin dyes and dye–TiO2 complexes, will be presented in the present work.
2. Computational details
All calculations were implemented using Gaussian 09 program.28 Firstly, we paid close attention to the selection of the most appropriate functional to get the vertical excitation energies and suitable HOMO and LUMO energy levels (HOMO is the highest occupied molecular orbitals and LUMO denotes the lowest unoccupied molecular orbitals) of dyes.3 The HOMO and LUMO levels of sensitizers were used to assess dye regeneration efficiency and electron injection efficiency. The ground-state geometry optimizations of four model compounds (NKX-2311, NKX-2677, NKX-2700 and NKX-2883) were performed in solvent ethanol using B3LYP/6-31G(d,p) level. The vertical excitation energies and energy levels of the four model systems were computationally explored using different functionals (including B3LYP,29 PBE0,30 CAM-B3LYP,31 LC-WPBE,32 M062X19 and WB97XD33) and the results were compared with experiments. Calculations showed that the most suitable excitation energies were obtained by the M062X functional (listed in Table 1S†) and the most fitting HOMO energy levels were got by the PBE0 functional (shown in Table 2S† along with the experimental data). Besides, the dependencies of HOMO level and transition energy on molecular geometry were explored using the six functionals mentioned above, and it showed that the best results (listed in Table 2S and Table 3S†) were obtained when ground-state geometries were optimized by the B3LYP functional. Therefore, for the eight newly designed coumarin dyes and the corresponding dye–TiO2 complexes, their UV/VIS spectra and HOMO/LUMO levels were respectively calculated by the M062X and PBE0 functionals based on the B3LYP optimized geometries. Solvation is essential for accurate representation of molecules' excitation energies in the realistic environment, thus the solvent effect (in ethanol) was taken into account using the polarized continuum model (PCM) for all calculations.34,35Then, the geometrical and electronic features of the free dyes were studied in detail. To investigate the efficiency of electron injection, both the compositions of those orbitals participated in the main electronic transitions and the driving force for electron injection were computed. The driving force for electron injection is related to the redox potentials which can be obtained by the Born–Haber cycle.36 The Born–Haber cycle is connected with the geometrical optimization of the mono-valent and bi-valent oxidized coumarin sensitizers. All redox potentials involved in our study were obtained versus the normal hydrogen electrode (NHE). Furthermore, to assess the efficiency of charge separation, we computed the electron density difference of electron transitions. The electron densities of all orbitals related to the electron transitions were calculated with code Multwfn 2.5.37 The extent of charge separation was quantified by the three parameters mentioned above, that is, the charge transfer distance (L), the overlap (Ω) between the zones of density depletion and increment, and the amount of transferred charge (Δe), which were calculated by DctViaCube software suit.26
In order to study the dye–titania complexes, we selected a neutral, stoichiometric cluster of Ti16O32, by exposing anatase 101 surface reported in Persson's work,38 which is enough large to reproduce the electronic absorption spectra of the complexes. In this case a mixed basis set (standard LANL2DZ basis set for Ti atoms and 6-31G(d,p) basis set for C, N, S, H and O atoms) was used to perform geometry optimizations and TD-DFT calculations. Moreover, to evaluate the coupling interaction between dye and TiO2 surface, the partial and total densities of states (PDOS and DOS) for the optimized complexes were investigated at the same theoretical level.
3. Results and discussion
The overall power conversion efficiency (η) can be characterized by the short-circuit current density (Jsc), the open circuit voltage (Voc), and the fill factor of cell (FF), the η can be expressed as:2
 | (1) |
| Jsc ∝ φLHE·φcc·φinject·φreg | (2) |
In present work, we started discussion about the two question mentioned above from two aspects (Jsc (including φLHE, φcc, φinject and φreg) and Voc).
3.1 How do different electron-deficient units and their number affect the performance of isolated dyes?
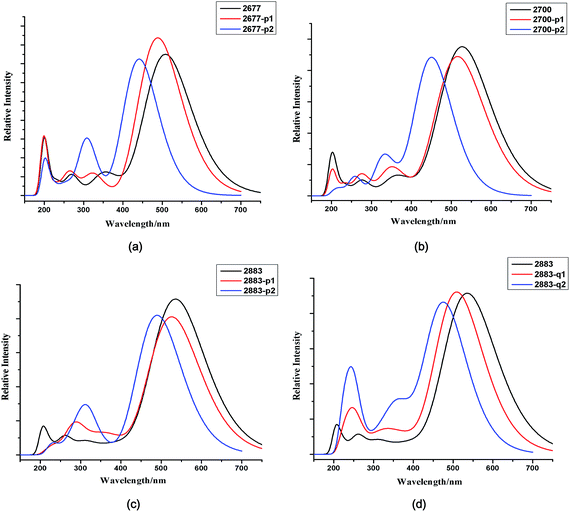
a. Absorption spectra. The simulated UV/VIS spectra of new designed dyes are presented in Fig. 2, and the related electronic transition data of all dyes are given in Table 4S.† Apparently, all dyes exhibit two major absorption bands appearing at 400–600 nm (B1 band) and 200–400 nm (B2 band), respectively. The absorption band at 200–400 nm is ascribed to a localized π–π* transition and the prominent band at 400–600 nm is attributed to the intramolecular charge transfer (ICT) excitation from donor to acceptor.
Calculated results shown that all the newly designed dyes with single electron-deficient unit exhibit a wider and stronger B2 (200–400 nm) band compared with the model sensitizers. The maximum absorption peaks (λmax) and light capturing ability of dyes are almost unchanged with one electron-deficient unit adopted. Taking NKX-2677 and NKX-2677-P1 as example, for B1 band, the λmax of NKX-2677-P1 is slightly blue-shifted by about 19 nm as compared with the reference dye NKX-2677. Moreover, light-harvesting efficiency of NKX-2677 and NKX-2677-P1 are 0.986 and 0.991, respectively. Obviously, the slight blue shift and the almost unchanged light capturing ability indicate the photophysical properties in B1 band for coumarin dyes could be maintained with one electron-deficient unit adopted. For B2 band, the location of absorption peak (λ*) for NKX-2677 is computed to be about 199 nm with oscillator strength f = 0.320. In comparison with NKX-2677, the NKX-2677-P1 displays a quite stronger absorption band at 199 nm with f = 0.557. Remarkably, the adoption of pyrimidine unit significantly enhances the absorption intensity of B2 band, and further increases the light-harvesting efficiency, φLHE (0.521 for NKX-2677 and 0.723 for NKX-2677-P1, as listed in Table 5S†). Accordingly, the adoption of electron-deficient unit greatly improves absorption properties in short-wavelength region with maintaining the intense absorption in long-wavelength region, which further increases the overall φLHE of dyes. Moreover, owing to stronger electron withdrawing ability, pyrimidine unit is more favorable to filling the optical absorption vacancy in short-wavelength region of coumarin sensitizers relative to quinoline unit.
b. Electronic structures and photoinduced charge transfer character. A favorable charge separation will facilitate electronic injection from excited dye to semiconductor surface, and then promote the photogenerated charge to be transported to the electrodes.2 Consequently, effective electron collection efficiency (φcc) requires a good intramolecular charge separation,40 so we evaluated φcc with molecular orbital distribution and three parameters (L, Ω and Δe), respectively.26
Generally, the sensitizers with push–pull structure are composed of an electron donor and an electron acceptor connected with conjugated π-spacer. In these systems, a photon absorption induces an electron transfer from the D part to the A part to form a D+–π–A− excited state. Ideally, the electron completely transfers from the donor and localizes on the acceptor upon excitation. But in most real cases, the transferred charge is delocalized from the zone in the vicinity of the donor to region nearby the acceptor, which makes the extent of charge separation is different. For DSSCs, the η would be decreased due to the incomplete charge separation, so quantifying the length and magnitude of the CT is of much concern to evaluate the performance of sensitizer. In this work, three parameters of CT mentioned above are used to quantify the degree of electron transfer. The total electron densities of initial and final states and their centroids should be calculated.
The CT distance (L) is defined by two barycenters of the electronic density depletion (R−) and increment (R+) zones upon excitation.
| L = |R+ − R−| | (3) |
The barycenters of electronic density depletion (R−) region and the electronic density increment (R+) region associated with electronic transition should be defined by ρ+(r) and ρ−(r):
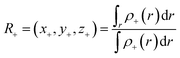 | (4) |
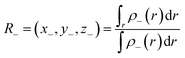 | (5) |
To calculate the two barycenters, the increase ρ+(r)/decrease ρ−(r) of the density due to the electronic transition is expressed as:
 | (6) |
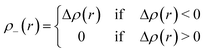 | (7) |
In eqn (6) and (7), Δρ(r) is defined as:
| Δρ(r) = ρES(r) − ρGS(r) | (8) |
 | (9) |
The overlap (Ω) between the zones of density depletion and increment is written as:
| Ω ∝ (L − H) | (10) |
To create an efficient charge-separated state, HOMO should be localized on the donor part, and LUMO should be localized on the acceptor.2 From Table 1, the introduction of single electron-deficient unit changes the charge population of HOMOs from entire molecules to donor groups of sensitizers, which leads to effective charge-separated state for coumarin sensitizer. To illustrate visually the electron transition, the electron density difference plots of S0 → S1 excitation and the three parameters were calculated (Table 1). In electron density difference plots, the direction of electron transfer is from the green area to the blue one. Clearly, the adoption of single pyrimidine or quinoline promotes more charge transfer to the acceptor part of dyes, and further increases the values of Δe and L, such as 1.100e− (NKX-2883) < 1.050e− (NKX-2883-Q1) < 1.120e− (NKX-2883-P1) and 9.403 Å (NKX-2883) < 10.405 Å (NKX-2883-P1) < 11.782 Å (NKX-2883-Q1). It indicates that adding one pyrimidine or quinoline unit into dye contributes to the formation of effective charge-separated state. In addition, the overlap (Ω) between the zones of density depletion and increment is also an important parameter to determine whether an effective charge separation between the donor and acceptor occurs or not. The weak overlap is accompanied by a good charge separation. As shown in Table 1, with one pyrimidine or quinoline adopted, the HOMO electron density of single electron-deficient unit tailored dyes is mainly localized on the coumarin 343 moiety (the donor part), which decreases the values of overlap (Ω), the calculated overlap (Ω) complies with the order of ΩNKX-2883-P1 (0.192) < ΩNKX-2883-Q1 (0.216) < ΩNKX-2883 (0.350). Again, the present theoretical results suggested that the adoption of single electron-deficient unit (especially pyrimidine) is advantageous to the effective charge separation because of the weaker overlap.
c. Electron injection and regeneration for sensitizers. It is well known that the charge injection is one of the key electron transfer processes and is important for the performance of DSSCs devices.2 In present work, we estimate the efficiency of electron injection from three aspects, that is, the energy levels of the acceptor fragment (conjugated π-spacer and anchoring group) and the donor of sensitizer, the electronic coupling between chromophores and TiO2 surface, and driving force of electron injection.19 Also, dye regeneration is one of the most central electron transfer processes for the overall DSSCs performance in terms of both the reuse and long life expectancies of sensitizers. The regeneration efficiency can be evaluated by the driver force of dye regeneration. The driving forces depend strongly on the ground state oxidation potential of sensitizers (Eox(dye)).19
| Eox(dye) ≈ εHOMO + 4.44 eV | (11) |
In this paper, all calculated results are gained by the PBE0 functional. The calculated (Table 2S†) for NKX-2677, NKX-2700 and NKX-2883 are 0.900 eV, 0.810 eV and 1.022 eV, respectively. These results are well in agreement with the experimental values (0.91 eV for NKX-2677,22 0.82 eV for NKX-2700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23 and 0.97 eV for NKX-2883).24 The small discrepancy between theory and experiment (<0.1 eV) suggests that the present theoretical method is reliable.
23 and 0.97 eV for NKX-2883).24 The small discrepancy between theory and experiment (<0.1 eV) suggests that the present theoretical method is reliable.
In general, the electron transfer is described as that electron is supposed to move from the LUMO of coumarin to the LUMO of semiconductor tunneling through the LUMO of π-spacer.18 To estimate the efficiency of electron injection from dye to TiO2 surface (φinject), the energy levels of donor fragment (coumarin 343) and acceptor moiety (conjugated π-spacer and anchoring group) were calculated (Table 2). Obviously, the LUMO energy levels of all of the acceptor groups are located below the LUMO energy level of C343 (−1.503 eV), which leads the energy drops of LUMOdonor–LUMOacceptor for one-pyrimidine-bridged or one-quinoline-bridged dyes be larger than those of their parent analogues. It indicates that the introduction of electron-deficient unit is favorable for the charge transfer from C343 to acceptor (the π-spacer and anchoring group), and further increase the contributions from the anchoring group to LUMO of dyes (Table 3). As reported by Troisi et al.,41 the large contribution from anchoring group of dye to LUMO will effectively strengthen electronic coupling between sensitizer and conduction band of semiconductor. Strong electronic coupling42,43 promotes the electron injection from dye to titania. From Table 3, our calculated results show that the contributions from the anchoring group of new designed dyes with single electron-deficient unit are comparatively lager than the corresponding reference dyes, which means the electronic coupling between dye and TiO2 is effectively enhanced by modifying the π-spacer of coumarin dyes with electron-deficient units for efficient electron injection.Additionally, the thermodynamic driving force (ΔG0) for electron injection is an important parameter to characterize the efficiency of electron injection, which provides an evidence to screen promising sensitizers for DSSCs. The driving force can evaluate the electron injection efficiency related to the corresponding vertical excitation:18
| φinject ∝ ΔG0 | (12) |
| ΔG0 = ECB − ΔEver − Eredox | (13) |
| Molecule | εLUMO | εHOMO | εLUMOdonor–LUMOacceptor |
|---|---|---|---|
| Coumarin 343 | −1.503 | −5.512 | |
| Frag-2311 | −2.665 | −7.620 | 1.162 |
| Frag-2677 | −2.807 | −6.266 | 1.304 |
| Frag-2677-P1 | −2.985 | −6.842 | 1.482 |
| Frag-2677-P2 | −3.110 | −6.978 | 1.607 |
| Frag-2700 | −2.839 | −5.927 | 1.336 |
| Frag-2700-P1 | −3.021 | −6.364 | 1.518 |
| Frag-2700-P2 | −3.108 | −6.938 | 1.605 |
| Frag-2883 | −3.004 | −6.259 | 1.501 |
| Frag-2883-P1 | −3.151 | −6.757 | 1.648 |
| Frag-2883-P2 | −3.229 | −7.375 | 1.726 |
| Frag-2883-Q1 | −3.022 | −6.490 | 1.519 |
| Frag-2883-Q2 | −2.966 | −6.589 | 1.463 |
| System | Orbital | Anchoring ligand | π-Linker | C343 |
|---|---|---|---|---|
| NKX-2311 | HOMO | 11 | 12 | 77 |
| LUMO | 35 | 20 | 46 | |
| NKX-2677 | HOMO | 4 | 34 | 62 |
| LUMO | 39 | 49 | 12 | |
| NKX-2677-P1 | HOMO | 2 | 28 | 70 |
| LUMO | 44 | 46 | 10 | |
| NKX-2677-P2 | HOMO | 0 | 8 | 92 |
| LUMO | 48 | 47 | 4 | |
| NKX-2700 | HOMO | 3 | 43 | 54 |
| LUMO | 35 | 56 | 9 | |
| NKX-2700-P1 | HOMO | 3 | 33 | 64 |
| LUMO | 42 | 49 | 6 | |
| NKX-2700-P2 | HOMO | 0 | 10 | 90 |
| LUMO | 45 | 55 | 3 | |
| NKX-2883 | HOMO | 3 | 39 | 58 |
| LUMO | 24 | 56 | 19 | |
| NKX-2883-P1 | HOMO | 1 | 31 | 68 |
| LUMO | 35 | 54 | 11 | |
| NKX-2883-P2 | HOMO | 0 | 19 | 80 |
| LUMO | 41 | 52 | 7 | |
| NKX-2883-Q1 | HOMO | 1 | 34 | 65 |
| LUMO | 34 | 56 | 10 | |
| NKX-2883-Q2 | HOMO | 0 | 24 | 76 |
| LUMO | 42 | 54 | 4 |
Calculated driving forces of electron injection43 for all sensitizers are also calculated and listed in Table 4. Introducing single pyrimidine or quinoline into dyes can increase the driving force, and further make for electron injection. For 2677 and NKX-2677-P1, the value of ΔG0 for NKX-2677-P1 (−2.543 eV) is more negative than that of NKX-2677 (−2.290 eV), it suggests that introducing single pyrimidine into dyes can increase the driving force for electron injection, and further make for electron injection. As shown in Table 4, all the newly designed sensitizers with single electron-deficient units have more negative ΔG0 than their corresponding parent compounds. Especially, both the electron injection driving forces of NKX-2883-P1 and NKX-2883-Q1 are more negative than that of NKX-2883 (the order is NKX-2883 (−2.210 eV) > NKX-2883-Q1 (−2.219 eV) > NKX-2883-P1 (−2.510 eV)). All results imply the introduction of single electron-deficient unit (especially pyrimidine unit) is conductive to improving the φinject of dyes.
| Scheme | ΔG(aq)a | ΔG0b | Eredoxc | ΔEverd | Eox(dye)e | Eox(dye*)f | ΔGregg | fh | LHEi |
|---|---|---|---|---|---|---|---|---|---|
| a Calculated Gibbs free energy change (ΔG(aq), in V) due to the oxidation of the dyes in aqueous solutions.b The driving force (ΔG0, in V) electron injection related to the electronic transition in B1 band.c Redox potential (Eredox vs. NHE, in V).d The vertical excitation energy (ΔEver, eV) corresponding to the maximum wavelength of spectral absorption.e The oxidized potential (vs. NHE, Eox(dye), in eV) of ground state.f The oxidized potential of the first excited state (Eox(dye*)) following unrelax path for dyes.g The regeneration energy (ΔGreg, in V).h The oscillation strength of the two major absorption bands (B1 and B2 bands).i The light harvest efficiency (LHE) corresponding to B1/B2 bands. Here the NHE is declared to be zero at all temperatures and its Gibbs free energy change takes the standard value of −4.44 eV. | |||||||||
| NKX-2311 | 10.302 | −2.894 | 5.862 | 2.528 | −1.131 | −1.397 | −0.831 | 1.626/0.345 | 0.976/0.548 |
| NKX-2677 | 9.216 | −2.290 | 4.776 | 2.047 | −0.900 | −1.147 | −0.600 | 1.407/0.614 | 0.961/0.757 |
| NKX-2677-P1 | 9.510 | −2.543 | 5.070 | 2.087 | −0.965 | −1.123 | −0.665 | 1.472/0.742 | 0.966/0.819 |
| NKX-2677-P2 | 10.087 | −3.070 | 5.647 | 2.138 | −1.078 | −1.059 | −0.778 | 0.911/0.933 | 0.877/0.883 |
| NKX-2700 | 8.890 | −2.048 | 4.450 | 1.962 | −0.810 | −1.152 | −0.510 | 1.825/0.710 | 0.985/0.805 |
| NKX-2700-P1 | 9.241 | −2.436 | 4.801 | 1.925 | −0.896 | −1.028 | −0.596 | 1.575/0.891 | 0.973/0.872 |
| NKX-2700-P2 | 9.674 | −2.704 | 5.234 | 2.089 | −1.055 | −1.034 | −0.755 | 1.080/1.305 | 0.917/0.951 |
| NKX-2883 | 9.089 | −2.210 | 4.649 | 1.998 | −1.022 | −0.976 | −0.722 | 1.900/0.361 | 0.987/0.564 |
| NKX-2883-P1 | 9.380 | −2.510 | 4.940 | 1.990 | −1.054 | −0.936 | −0.754 | 1.568/0.501 | 0.973/0.685 |
| NKX-2883-P2 | 9.891 | −2.889 | 5.451 | 2.122 | −1.179 | −0.944 | −0.879 | 1.371/0.653 | 0.957/0.778 |
| NKX-2883-Q1 | 9.140 | −2.219 | 4.700 | 2.042 | −1.046 | −0.995 | −0.746 | 1.695/0.577 | 0.980/0.735 |
| NKX-2883-Q2 | 9.192 | −2.068 | 4.752 | 2.244 | −1.099 | −1.145 | −0.799 | 1.430/0.668 | 0.963/0.785 |
The regeneration of sensitizers is also considered in the present research, and it can be estimated by the driver force of dye regeneration (ΔGreg).18
| ΔGreg = Eox(dye) + 0.3 | (14) |
As listed in Table 4, the regeneration driving forces are NKX-2677 (−0.600 eV) > NKX-2677-P1 (−0.665 eV) and NKX-2700 (−0.510 eV) > NKX-2700-P1 (−0.596 eV), indicating that the adoption of single pyrimidine unit is beneficial to the dye regeneration. For NKX-2883-P1 and 2883-Q1, the values of regeneration driving forces also become more negative with the introduction of electron-deficient unit and the order of driving force for dye regeneration is NKX-2883 (−0.722 eV) > NKX-2883-Q1 (−0.746 eV) > NKX-2883-P2 (−0.754 eV). All calculated results imply the driving forces for dye regeneration should be more negative by adopting electron-deficient unit (see Table 4).
d. The open circuit voltage. For our investigated dyes, the adoption of electron-deficient unit slightly affects the shift of conduction band edge of semiconductor, so the number of injected electron in the conduction band (nc) is the main factor that influence the values of Voc. As reported by Kusama et al.,44 injected electron in conduction band of TiO2 could recombine with electron acceptor (I2) and oxidized sensitizers leading to small nc. It is accepted that I2 preferred to bind with N of CN, S of thiophene forming dye–iodine complexes available for serious charge recombination, which is the main route of photovoltage losses.16,45 In this work, the absence of sulfur atom in electron-deficient unit (pyrimidine and quinoline) for NKX-2677-P1, NKX-2700-P1, NKX-2883-P1 and NKX-2883-Q1 could be beneficial to suppress charge recombination, which improves the nc for high Voc.
a. Absorption spectra. Similarly, the UV-VIS absorption spectra of NKX-2677-P2, NKX-2700-P2, NKX-2883-P2 and NKX-2883-Q2 also exhibit two major absorption (B1 and B2) bands. The maximum absorption peak (λmax) of NKX-2677-P2 adopted the second pyrimidine unit blue-shifts to 441 nm from 489 nm of NKX-2677-P1 (see Table 4S†) which could be explained by the fact that the dihedral angle formed between the π-spacer and coumarin 343 of NKX-2677-P2 is more than 10°, whereas the dihedral angle of NKX-2677-P1 is less than 3°. NKX-2677-P2 displays a stronger and red-shifted B2 absorption band (λ*) comparing with NKX-2677-P1. As shown in Table 4S,† the M062X functional simulated B2 band is red-shifted with inserting a second pyrimidine moiety from NKX-2677-P1 to NKX-2677-P2 by 102 nm, and their corresponding oscillation strengths are 0.557 and 0.678, respectively. Thus, the calculated φLHE of B2 band increases with the growing number of pyrimidine unit (0.723 for NKX-2677-P1 and 0.790 for NKX-2677-P2). Obviously, the two pyrimidine or quinoline units in these four dyes destroy the planarity of molecular backbone, which results in a significant blue-shifted B1 band. However, the situation of B2 band is just opposite to that of B1 band, that is, the introduction of a couple of pyrimidine or quinoline units effectively shifts the absorption peaks (λ*) of B2 band to longer wavelength region and significantly enhances the corresponding absorption intensity. Comprehensively, we considered that increasing the amount of electron-deficient units is unfavorable for improving the φLHE of coumarin dye due to the obviously blue shift of λmax.
b. Electronic structures and photoinduced charge transfer character. As shown in Table 1, the electron density distributions of HOMOs of the sensitizers with two electron-deficient groups are more centralized on the coumarin 343 than that of the dyes with single electron-deficient units, whereas all the sensitizers' LUMOs are localized on the linker and acceptor moieties. This reveals that the introduction of two electron-deficient units changes the charge population of HOMOs and induces the better charge separation. Also, the three important parameters (L, Δe and Ω) of CT are calculated and used to discuss the property of charge transfer for the new sensitizers. Our calculated results show that with increase of the number of electron-deficient pyrimidine the values of Δe and L are raised, whereas the value of the overlap is decreased. This means that the increase of pyrimidine would be helpful to improve the efficiency of charge separation. However, the overlap of NKX-2883-Q2 is larger than that of NKX-2883-Q1 (although they are still smaller than that of NKX-2883, the order is ΩNKX-2883-Q1 (0.2162) < ΩNKX-2883-Q2 (0.2410) < ΩNKX-2883 (0.3495), which indicates the adoption of single quinoline favors charge separation, but the introduction of couple of quinoline units could not further improve the efficiency of charge separation. Thus, the increasing number of quinoline is not suitable to improve the performance of coumarin-based DSSCs. The reason that pyrimidine unit motivates more effective charge separation than quinoline group may come from the following facts: better coplanarity of pyrimidine with their neighboring groups and its stronger electron withdrawing ability.
c. Electron injection and regeneration for sensitizers. From Table 2, evidently, the LUMO energy levels of all the acceptor groups with two pyrimidine units are located below those of all the acceptor moieties with single pyrimidine, therefore the energy drop of LUMOdonor–LUMOacceptor would be effectively increased with more pyrimidine unit adopted. It suggests that the adoption of more pyrimidine units is favorable for charge separation between the C343 donor and the extended acceptor (the π-spacer and anchoring group) and further increases the contribution from the anchoring group to the LUMO of dye (Table 3). For example, the contributions for NKX-2883-P1 and NKX-2883-P2 respectively are 35% and 41%, which indicates that NKX-2883-P2 has stronger electronic coupling between sensitizers and TiO2 surface for effective electron injection. Also, as shown in Table 4, the more the adopted pyrimidine units, the higher the driving force of electron injection would be. However, due to the weaker electron withdrawing ability of quinoline unit and poor coplanarity of multiple quinoline-bridged dyes, it should be pointed out that the energy drop of 2883-Q2 (1.436 eV) is slightly smaller than that of 2883-Q1 (1.459 eV) and the values of ΔG0(dye) become more negative with more quinoline introduced into coumarin dyes (−2.219 eV (NKX-2883-Q1) > −2.068 eV (NKX-2883-Q2)), which implies the introduction of more quinoline units weakens the charge separation and is not conductive to the electron injection process.
The driving force of dye regeneration (ΔGreg) is also calculated and is used to investigate the relationship between the amount of electron-deficient units and the efficiency of dye regeneration. As displayed in Table 4, the absolute values of driving forces for dye regeneration are increased with more pyrimidine or quinoline unit adopted, which means that increasing the amount of pyrimidine and quinoline units could promote the regeneration of oxidized dyes.46,47 The effective regeneration of oxidized dyes is beneficial to the unidirectional charge transport and avoiding the charge recombination.48 Alebbi49 pointed out that inefficient regeneration could limit the device photocurrent. Therefore, the present theoretical results reveal that the sensitizers combining two electron-deficient groups should have a vantage of regeneration of oxidized dyes.
3.2 How do different electron-deficient units and their number affect electronic structures and absorption spectra of dye–TiO2 complexes?
So far, we have described how the electron-deficient units and their number affect the electronic and structural properties of free coumarin sensitizers, but it is not sufficient to look at the electronic structures of these systems individually. Thus, the next step in this contribution was to investigate the dye–titania complexes2,15,50 (including their electronic structures, absorption spectra, and electronic coupling between dye and TiO2). FT-IR8,18 study suggested that the sensitizers are chemically adsorbed on the surface of nanocrystalline TiO2 in a bidentate carboxylate mode. In this bidentate structure, both the carboxylic oxygen atoms are bound to two five-fold coordinated Ti atoms, and the acid hydrogen atom (H) is bound to the bridging oxygen on the TiO2 surface. Therefore we adopted the bidentate bridging mode for adsorption of all of the sensitizers on the TiO2 nanoparticle surface. The optimized geometries for the four dye–TiO2 complexes are given in Fig. 3. Generally, upon excitation, charge injection from sensitizer to semiconductor can occur through direct mechanism or indirect mechanism.50,51 The direct mechanism is a “one-step” electron injection from the ground state of sensitizer to the conduction band of semiconductor. In the direct-mechanism case, a new charge-transfer band should appear in the absorption spectrum, but it is not present in the spectra of isolated sensitizer and semiconductor. In indirect mechanism, the charge is firstly provoked from the ground state of sensitizer to excited state which is also localized on dye itself, and then the charge transfers from excited state into the conduction band. The mechanism of electron injection plays a vital role in determining η of DSSCs. In the following discussion, we focused on these five dye–TiO2 complexes (including NKX-2883/TiO2, NKX-2883-P1/TiO2, NKX-2883-P2/TiO2, NKX-2883-Q1/TiO2 and NKX-2883-Q2/TiO2 complexes) to probe how the different electron-deficient units and their number affect the performance of these newly designed dye–titania complexes. | ||
| Fig. 3 Optimized geometries for the eight dye–TiO2 complexes and their HOMO and LUMO orbitals relevant during photoexcitation. | ||
For all dye–TiO2 complexes, the absorption properties are closely related to those of their free dyes. As presented in Fig. 1S–3S,† it is found that a broad and enhanced B2 absorption is obtained for all the dye–titania complexes with single electron-deficient unit, which fills the optical absorption vacancy in short-wavelength region and thus improves the photo-to-electric efficiency of coumarin-based DSSCs. While for those dye–TiO2 complexes with two electron-deficient groups, the efficiency is not significantly enhanced due to the seriously blue-shifted absorption in long-wavelength region, although the oscillator strength of B2 band is also strengthened.
Several photo-excitations are involved in the formation of absorption spectra of these five complexes. A few of these excitations showing high oscillator strengths are given in Table 4S.† Most of these excitations are mainly originated from HOMO to HOMO-2. The unoccupied orbitals involved in the major excitations mainly come from LUMO (Table 4S†). For these five complexes, the change in electronic structure upon adsorption is small, which results in the energy of isolated photoexcited dyes to reside near the energy of excited states of dye–titania complexes. Thus, LUMOs of dye–titania complexes are very similar to those of free dyes, and therefore localized mainly on dyes, which means weak electronic coupling between dyes and TiO2.2 As reported in previous literature,15 the electronic coupling between semiconductor conduction band and dye molecule strongly influences the electron injection mechanism. The direct mechanism requires a strong electronic coupling, while weak electronic coupling favors the indirect mechanism. It suggests that in the present cases the indirect mechanism should be more possible. Besides, the different number of pyrimidine and quinoline units only has a small effect on the electronic structures of these dye–titania complexes.
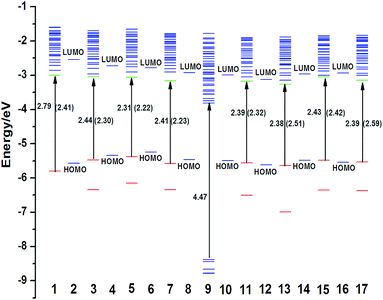
After binding to titanium there is a redistribution of energy levels.39 The computed Kohn–Sham orbital energy levels for isolated dyes, (TiO2)16 cluster and dye–TiO2 complexes are presented in Fig. 4. As reported in previous studies, the higher the LUMO is located in the semiconductor conduction band, the more efficient the electron injection is. From Fig. 4, LUMOs of NKX-2883-P1/TiO2 and NKX-2883-Q1/TiO2 are localized higher than that of NKX-2883/TiO2, which implies the introduction of single pyrimidine or quinoline unit contributes to providing more sufficient driving force for electron injection. However, compared with NKX-2883-P1/TiO2 complex, the location of LUMO for NKX-2883-P2/TiO2 complex is almost unchanged, revealing that they have almost the same electron injection efficiency. Introducing one more quinoline unit into NKX-2883-Q1/TiO2 makes the location of LUMO for NKX-2883-Q2/TiO2 is lower, and therefore decreases the efficiency of electron injection, which accords with our discussed results mentioned above. To better predict the efficiency of electron injection, we should adopt the molecular dynamics of the ultrafast electron injection in our further research.52–54
The total and partial densities of states18 for dye–TiO2 complexes were used to illustrate electronic coupling between the dye and cluster, which are showed in Fig. 4S.† For all dye–titania complexes, the electronic densities of LUMO orbitals are mainly localized on the dyes with slight contribution on cluster, it suggests weak electronic coupling between dye and TiO2. This small contribution plays a key role in heterogeneous electron transfer. From Fig. 4S,† we clearly observe that with single pyrimidine and quinoline units adopted into NKX-2883/TiO2 complex, this small contribution is increased slightly, which indicates more mixing between dye and cluster orbitals leading to slightly enhanced electron coupling between dye and TiO2. With one more pyrimidine or quinoline units adopted into NKX-2883-P1/TiO2 and NKX-2883-Q1/TiO2 complexes, the contributions from the cluster to unoccupied molecular orbitals is almost unaltered, it implies the interfacial interaction between dye and TiO2 cluster would be insensitive by increasing the number of pyrimidine or quinoline units.
In the present work, only two pyrimidine and quinoline units are used to study the number of electron-deficient units how to influences the performance of sensitizers and DSSCs. We believe it is enough because it has been found that the introduction of two electron-deficient units seems unfavorable for improving the photophysical properties of dye–TiO2 systems. Additionally, in our previous report,19 we have proved that the extension the length of the π-spacer is disadvantageous to increase the performance of DSSCs.
4. Conclusions
In summary, we offered a comprehensive discussion about the influence of different electron-deficient units and their amount on absorption property, charge separation, electron injection and dye regeneration. Briefly, the Jsc (φLHE, φcc, φinject and φreg) and Voc can be effectively improved by adopting single pyrimidine or quinoline unit into model coumarin dyes. Moreover, owing better coplanarity with the neighboring groups and its stronger electron withdrawing ability, pyrimidine should be more suitable to improve the performance of DSSCs relative to quinoline. Further, with a second electron-deficient unit adopted, a red-shifted and strengthened absorption in short-wavelength region and a blue-shifted absorption in long-wavelength region are obtained. The introduction of more pyrimidine units facilitates the electron transfer from donor to acceptor and favors the effective electron injection, whereas the second quinoline displays opposite effect. Despite the introduction of electron-deficient unit makes for electronic coupling between dyes and TiO2, but cannot alter the indirect mechanism of electron injection for these investigated systems. The present theoretical exploration shows that the performance of metal-free coumarin sensitizers and the efficiency of the DSSCs are sensitive to the amount and variety of electron-deficient units. We believe it is a promising future research direction to broaden and strengthen the absorption in short-wavelength region (B band) of metal-free coumarin sensitizer and further improve the efficiency of the DSSCs.Acknowledgements
We acknowledge generous financial support from the National Natural Science Foundation of China (no. 21173169 and 20803059), Chongqing Municipal Natural Science Foundation (cstc2013jcyjA90015) and the Fundamental Research Funds for the Central Universities (no. DXJK2013A008).Notes and references
- B. O'regan and M. Grfitzeli, nature, 1991, 353, 737–740 CrossRef.
- A. Hagfeldt, G. Boschloo, L. Sun, L. Kloo and H. Pettersson, Chem. Rev., 2010, 110, 6595–6663 CrossRef CAS PubMed.
- M. Grätzel, J. Photochem. Photobiol., C, 2003, 4, 145–153 CrossRef.
- M. Grätzel, J. Photochem. Photobiol., A, 2004, 164, 3–14 CrossRef.
- M. K. Nazeeruddin, F. De Angelis, S. Fantacci, A. Selloni, G. Viscardi, P. Liska, S. Ito, B. Takeru and M. Grätzel, J. Am. Chem. Soc., 2005, 127, 16835–16847 CrossRef CAS PubMed.
- N. Martsinovich and A. Troisi, Energy Environ. Sci., 2011, 4, 4473–4495 CAS.
- R. R. Frontiera, J. Dasgupta and R. A. Mathies, J. Am. Chem. Soc., 2009, 131, 15630–15632 CrossRef CAS PubMed.
- S. Agrawal, P. Dev, N. J. English, K. R. Thampi and J. MacElroy, J. Mater. Chem., 2011, 21, 11101–11108 RSC.
- G. Reynolds and K. Drexhage, Opt. Commun., 1975, 13, 222–225 CrossRef CAS.
- R. Sánchez-de-Armas, M. Á. San Miguel, J. Oviedo and J. F. Sanz, Phys. Chem. Chem. Phys., 2012, 14, 225–233 RSC.
- A. Yella, H.-W. Lee, H. N. Tsao, C. Yi, A. K. Chandiran, M. K. Nazeeruddin, E. W.-G. Diau, C.-Y. Yeh, S. M. Zakeeruddin and M. Grätzel, Science, 2011, 334, 629–634 CrossRef CAS PubMed.
- K. Hara, T. Sato, R. Katoh, A. Furube, Y. Ohga, A. Shinpo, S. Suga, K. Sayama, H. Sugihara and H. Arakawa, J. Phys. Chem. B, 2003, 107, 597–606 CrossRef CAS.
- Z.-S. Wang, Y. Cui, Y. Dan-oh, C. Kasada, A. Shinpo and K. Hara, J. Phys. Chem. C, 2008, 112, 17011–17017 CAS.
- X. Zhang, J.-J. Zhang and Y.-Y. Xia, J. Photochem. Photobiol., A, 2008, 194, 167–172 CrossRef CAS.
- R. Sánchez-de-Armas, M. A. San-Miguel, J. Oviedo and J. F. Sanz, J. Chem. Phys., 2012, 136, 194702 CrossRef PubMed.
- Y. Wu and W. Zhu, Chem. Soc. Rev., 2013, 42, 2039–2058 RSC.
- L.-Y. Lin, C.-H. Tsai, K.-T. Wong, T.-W. Huang, C.-C. Wu, S.-H. Chou, F. Lin, S.-H. Chen and A.-I. Tsai, J. Mater. Chem., 2011, 21, 5950–5958 RSC.
- M. Guo, R. He, Y. Dai, W. Shen, M. Li, C. Zhu and S. H. Lin, J. Phys. Chem. C, 2012, 116(16), 9166–9179 CAS.
- M. Guo, M. Li, Y. Dai, W. Shen, J. Peng, C. Y. Zhu, S. H. Lin and R. X. He, RSC Adv., 2013, 3, 17515–17526 RSC.
- K. Hara, Z.-S. Wang, T. Sato, A. Furube, R. Katoh, H. Sugihara, Y. Dan-oh, C. Kasada, A. Shinpo and S. Suga, J. Phys. Chem. B, 2005, 109, 15476–15482 CrossRef CAS PubMed.
- X. Ren, Q. Feng, G. Zhou, C.-H. Huang and Z.-S. Wang, J. Phys. Chem. C, 2010, 114, 7190–7195 CAS.
- K. Hara, Y. Dan-oh, C. Kasada, Y. Ohga, A. Shinpo, S. Suga, K. Sayama and H. Arakawa, Langmuir, 2004, 20, 4205–4210 CrossRef CAS PubMed.
- Z.-S. Wang, Y. Cui, Y. Dan-oh, C. Kasada, A. Shinpo and K. Hara, J. Phys. Chem. C, 2007, 111, 7224–7230 CAS.
- Z. S. Wang, Y. Cui, K. Hara, Y. Dan-oh, C. Kasada and A. Shinpo, Adv. Mater., 2007, 19, 1138–1141 CrossRef CAS.
- H. Choi, H. Choi, S. Paek, K. Song, M.-s. Kang and J. Ko, Bull. Korean Chem. Soc., 2010, 31, 125–132 CrossRef CAS.
- I. Ciofini, T. Le Bahers, C. Adamo, F. Odobel and D. Jacquemin, J. Phys. Chem. C, 2012, 116, 11946–11955 CAS.
- T. Le Bahers, C. Adamo and I. Ciofini, J. Chem. Theory Comput., 2011, 7, 2498–2506 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson and H. Nakatsuji, et al., Gaussian 09, Revision A.01, Gaussian, Inc., Wallingford, CT, 2009 Search PubMed; J. Preat, C. Michaux, D. Jacquemi and H. Nakatsuji, Gaussian 09, Revision A.01 edn, Gaussian Inc., Wallingford CT, 2009 Search PubMed.
- Y. Kurashige, T. Nakajima, S. Kurashige, K. Hirao and Y. Nishikitani, J. Phys. Chem. A, 2007, 111, 5544–5548 CrossRef CAS PubMed.
- T. Le Bahers, F. Labat, T. Pauporté, P. P. Lainé and I. Ciofini, J. Am. Chem. Soc., 2011, 133, 8005–8013 CrossRef CAS PubMed.
- P. Dev, S. Agrawal and N. J. English, J. Phys. Chem. A, 2013, 117, 2114–2124 CrossRef CAS PubMed.
- I. H. Nayyar, E. R. Batista, S. Tretiak, A. Saxena, D. L. Smith and R. L. Martin, J. Chem. Theory Comput., 2013, 9, 1144–1154 CrossRef CAS.
- F. Lachaud, C. Jeandon, M. Beley, R. Ruppert, P. C. Gros, A. Monari and X. Assfeld, J. Phys. Chem. A, 2012, 116, 10736–10744 CrossRef CAS PubMed.
- D. Maftei, G. Zbancioc, I. Humelnicu and I. Mangalagiu, J. Phys. Chem. A, 2013, 117, 3165–3175 CrossRef CAS PubMed.
- F. Lipparini and V. Barone, J. Chem. Theory Comput., 2011, 7, 3711–3724 CrossRef CAS.
- J. Wang, F.-Q. Bai, B.-H. Xia, L. Feng, H.-X. Zhang and Q.-J. Pan, Phys. Chem. Chem. Phys., 2011, 13, 2206–2213 RSC.
- T. Lu and F. Chen, J. Chem. Theory Comput., 2012, 33, 580–592 CAS.
- M. Nilsing, P. Persson and L. Ojamäe, Chem. Phys. Lett., 2005, 415, 375–380 CrossRef CAS.
- A. V. Akimov, A. J. Neukirch and O. V. Prezhdo, Chem. Rev., 2013, 113, 4496–4565 CrossRef CAS PubMed.
- S. Nachimuthu, C.-M. Lo and J.-C. Jiang, J. Phys. Chem. Lett., 2013, 4, 524–530 CrossRef PubMed.
- D. R. Jones and A. Troisi, Phys. Chem. Chem. Phys., 2010, 12, 4625–4634 RSC.
- M. Pastore and F. D. Angelis, ACS Nano, 2009, 4, 556–562 CrossRef PubMed.
- J. M. Rehm, G. L. McLendon, Y. Nagasawa, K. Yoshihara, J. Moser and M. Grätzel, J. Phys. Chem., 1996, 100, 9577–9578 CrossRef.
- H. Kusama and H. Sugihara, J. Comput. Chem., 2005, 26, 1372–1382 CrossRef CAS PubMed.
- W. Li, Y. Wu, Q. Zhang, H. Tian and W. Zhu, ACS Appl. Mater. Interfaces, 2012, 4, 1822–1830 CAS.
- S. M. Feldt, P. W. Lohse, F. Kessler, M. K. Nazeeruddin, M. Gratzel, G. Boschloo and A. Hagfeldt, Phys. Chem. Chem. Phys., 2013 Search PubMed.
- T. Daeneke, A. J. Mozer, T.-H. Kwon, N. W. Duffy, A. B. Holmes, U. Bach and L. Spiccia, Energy Environ. Sci, 2012, 5, 7090–7099 CAS.
- J. N. Clifford, E. Palomares, M. K. Nazeeruddin, M. Grätzel, J. Nelson, X. Li, N. J. Long and J. R. Durrant, J. Am. Chem. Soc., 2004, 126, 5225–5233 CrossRef CAS PubMed.
- M. Alebbi, C. A. Bignozzi, T. A. Heimer, G. M. Hasselmann and G. J. Meyer, J. Phys. Chem. B, 1998, 102, 7577–7581 CrossRef CAS.
- S. Agrawal, P. Dev, N. J. English, K. R. Thampi and J. MacElroy, Chem. Sci., 2012, 3, 416–424 RSC.
- R. o. Sánchez-de-Armas, J. Oviedo, M. A. n. San Miguel and J. F. Sanz, J. Phys. Chem. C, 2011, 115, 11293–11301 Search PubMed.
- R. Long and O. V. Prezhdo, J. Am. Chem. Soc., 2011, 133, 19240–19249 CrossRef CAS PubMed.
- D. P. Hoffman, O. P. Lee, J. E. Millstone, M. S. Chen, T. A. Su, M. Creelman, J. M. Frechet and R. A. Mathies, J. Phys. Chem. C, 2013, 117, 6990–6997 CAS.
- A. Listorti, B. O'Regan and J. R. Durrant, J. Mater. Chem., 2011, 23, 3381–3399 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed discussion and calculated results obtained by different functionals (BELYP, PBE0 or M062X). See DOI: 10.1039/c4ra07904c |
| This journal is © The Royal Society of Chemistry 2014 |