Hexanuclear [Ni2Ln4] clusters exhibiting enhanced magnetocaloric effect and slow magnetic relaxation†
Cai-Ming Liu*,
De-Qing Zhang and
Dao-Ben Zhu
Beijing National Laboratory for Molecular Sciences, Center for Molecular Science, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, P. R. China. E-mail: cmliu@iccas.ac.cn
First published on 3rd October 2014
Abstract
Two hexanuclear 3d–4f cluster complexes containing the pyridine-2-aldoximate ligand (pao−), [Ni2Ln4 (hfac)8(pao)6(CH3COO)2(MeOH)]·H2O·MeOH (Ln = Gd, 1; Ln = Dy, 2; hfac− = hexafluoroacetylacetonate), have been synthesized, which show similar structures with an interesting ‘trigonal bipyramid’ metal topology. Magnetic determinations revealed that the Ni–Gd species has a spin ground state of 16 due to the dominant ferromagnetic exchange, exhibiting an enhanced magnetocaloric effect, which can be used as a cryogenic magnetic cooler; while the Ni–Dy analogue displays slow magnetic relaxation, a typical characteristic of a single-molecule magnet.
Introduction
Recently, heterometallic 3d–4f cluster complexes have played an important role in the development of nanoscale molecular magnetic materials, especially for single-molecule magnets (SMMs)1 and low-temperature magnetic coolers.2 The SMMs possess many potential applications in fields such as quantum computing, magnetic information storage devices and spintronics,1,3 because they display slow magnetization relaxation and superparamagnet-like behaviors at low temperature. While the environmentally friendly low-temperature magnetic coolers are based on magnetocaloric effect (MCE), which is commonly evaluated by magnetic entropy change (−ΔSm).2,4 Interestingly, both the molecular magnetic materials require high spin ground states, which can be achieved by the ferromagnetic interaction between the 3d and 4f metal ions. Furthermore, the magneto-anisotropy is necessary for the SMMs whereas the magneto-isotropy favors the MCEs, therefore, it supplies a practical approach to the different nano-sized molecular magnetic materials, either the SMM or the low-temperature magnetic cooler, through change of the Ln3+ ions with different magneto-isotropy in the same 3d–4f cluster family. For examples, the Dy3+ ion is often used for the design of the SMMs, while the Gd3+ ion is an excellent element to construct molecular magnetic coolers.Up to now, more and more heterometallic Ni–Gd clusters have been designed to be low-temperature magnetic coolers,5 including very large nuclearity aggregates [Ni9Gd2],5a [Ni12Gd5],5d [Ni10Gd42]5c and [Ni12Gd36].5b However, still no enhanced MCEs were observed in these compounds. In addition, their Ni–Dy analogues seldom show slow magnetization relaxation, the main reason maybe is that the whole molecule's magneto-anisotropy of the heterometallic 3d–4f clusters is difficult to be controlled to display the SMM properties. Therefore, it is necessary to further design and synthesize new 3d–4f cluster family in which a switch from the SMM to the coolant (or vice versa) can be achieved via facile lanthanide ion changes. For the Ni–Ln cluster complexes, only two such systems have been reported recently.5c,i
We are also interested in the molecular nanomagnet materials.6 Recently, we used a salicylaldoximate ligand (Et-sao2−) to construct a heptanuclear Mn–Ln cluster system MnIII3MnIVO3Ln3(OH)(piv)6(EtO)3(EtOH)3(Et-sao)3 (Ln = Gd and Dy; piv = pivalate), whose magnetic properties could be adjusted to be the SMM or the cryogenic magnetic cooler, depending on the lanthanide ions used.7 As a continuation of the work, we chose another oxime ligand, pyridine-2-aldoximate (pao−, Scheme 1), to assembly the 3d–4f cluster system. Herein, we report the syntheses, crystal structures and magnetic properties of two novel hexanuclear Ni–Ln cluster complexes, [Ni2Ln4 (hfac)8(pao)6(CH3COO)2(MeOH)] ·H2O·MeOH (Ln = Gd, 1; Ln = Dy, 2; hfac− = hexafluoroacetylacetonate), which show a ‘trigonal bipyramid’ metal topology and ferromagnetic interactions. Interestingly, complex 1 displays an enhanced MCE while 2 shows slow magnetic relaxation.
Experimental
Materials and physical measurements
Unless otherwise mentioned, all reagents were commercially available and used as received. The starting materials Ni(paoH)2Cl2 and Ln(acac)2(CH3COO)(H2O)2 were prepared according to the literature methods.8,9 Elemental analyses were performed on a Varlo ELIII elemental analyzer. Infrared spectra were recorded on a TENSOR27 Bruker spectrophotometer in the region of 400–4000 cm−1 using the KBr pallet samples. The magnetic susceptibility measurements were carried out on a Quantum Design MPMS-XL5 SQUID magnetometer in the temperature range of 1.9–300 K. Diamagnetic corrections of all constituent atoms were estimated from Pascal's constants.10Preparation of 1
A mixture of Ni(paoH)2Cl2 (0.25 mmol), Gd(acac)2(CH3COO)(H2O)2 (0.25 mmol) and Et3N (0.5 mmol) in 10 ml of MeOH was sonicated for 10 min, a brown solution was formed, which was allowed to slowly evaporate for several days, giving brown block crystals. Yield 70% based on Gd. Elemental analysis (%) calculated for C82H54Gd4F48N12Ni2O29: C, 29.58; H, 1.63; N, 5.05; found: C, 29.53; H, 1.67; N, 5.01. IR (KBr, cm−1): 3429 (b, s), 3140 (w), 3072 (w), 1655 (s), 1608 (m), 1580 (m), 1555 (m), 1530 (s), 1511 (m), 1478 (m), 1443 (w), 1415 (w), 1347 (w), 1256 (s), 1208 (s), 1142 (s), 1099 (m), 1017 (w), 950 (w), 917 (w), 891 (w), 795 (m), 774 (w), 749 (w), 680 (w), 661 (m), 642 (w), 585 (m), 528 (w), 447 (w), 422 (w).Preparation of 2
Complex 2 was produced as brown block crystals by an approach similar to that of 1, except that Dy(acac)2(CH3COO)(H2O)2 was used instead of Gd(acac)2(CH3COO) (H2O)2. Yield 65% based on Dy. Elemental analysis (%) calculated for C82H54Dy4F48N12Ni2O29: C, 29.39; H, 1.62; N, 5.02; found: C, 29.43; H, 1.65; N, 4.99. IR (KBr, cm−1): 3444 (b, s), 3141 (w), 3075 (w), 1654 (s), 1608 (m), 1580 (m), 1555 (m), 1531 (s), 1511 (m), 1478 (m), 1444 (w), 1419 (w), 1386 (w), 1346 (w), 1257 (s), 1208 (s), 1144 (s), 1099 (m), 1017 (w), 951 (w), 921 (w), 891 (w), 797 (m), 775 (w), 749 (w), 681 (w), 662 (m), 642 (w), 586 (m), 528 (w), 447 (w), 422 (w).X-ray crystallography
The diffraction data were collected at 173(2) K on a Rigaku Saturn 724 diffractometer with monochromated Mo-Kα radiation (λ = 0.71073 Å). Lorentz-polarization effects and absorption corrections were applied. Direct methods were adopted to solve the structures, which were refined with full-matrix least-squares techniques using SHELXS-97 and SHELXL-97 programs.11 The non-hydrogen atoms were refined anisotropically, and the hydrogen atoms were added theoretically onto the attached atoms and refined isotropically with fixed thermal factors. Selected crystallographic data and structure determination parameters are given in Table 1.| Compound | 1 | 2 |
|---|---|---|
| a R1 = ∑||Fo| − |Fc||/∑|Fo|.b wR2 = ∑{[w(Fo2 − Fc2)2]/∑[wFo2]2}1/2. | ||
| Chemical formula | C82H54Gd4F48N12Ni2O29 | C82H54Dy4F48N12Ni2O29 |
| Formula weight | 3329.79 | 3350.79 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | P21/c | P21/c |
| a/Å | 16.176 (3) | 16.217 (3) |
| b/Å | 25.626 (5) | 25.446 (5) |
| c/Å | 28.494 (6) | 28.525 (6) |
| β/° | 103.74 (3) | 104.02 (3) |
| V/Å3 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 474 (4) 474 (4) |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 421 (4) 421 (4) |
| Z | 4 | 4 |
| T/K | 173 (2) | 173 (2) |
| λ(Mo-Kα)/Å | 0.71073 | 0.71073 |
| ρcalc/g cm−3 | 1.928 | 1.949 |
| μ(Mo-Kα)/mm−1 | 2.753 | 3.060 |
| θ range | 1.08° ≤ θ ≤ 25.02° | 1.09° ≤ θ ≤ 25.02° |
| Limiting indices | −18 ≤ h ≤ 19, −30 ≤ k ≤ 30, −33 ≤ l ≤ 33 | −19 ≤ h ≤ 19, −30 ≤ k ≤ 30, −33 ≤ l ≤ 33 |
| Reflections collected | 64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 729 729 |
68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 739 739 |
| Unique reflections | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 141 141 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 030 030 |
| R1a [I > 2σ(I)] | 0.0741 | 0.0703 |
| wR2b [I > 2σ(I)] | 0.1514 | 0.1432 |
| R1a [all data] | 0.0869 | 0.0821 |
| wR2b [all data] | 0.1583 | 0.1493 |
| S [I > 2σ(I)] | 1.156 | 1.156 |
| S [all data] | 1.160 | 1.156 |
Results and discussion
Synthesis
It is well known that the polydentate ligand salicylaldoxime (R-saoH2, R = H, Me, Et, Ph etc.) has been extensively employed to synthesize a series of [Mn3] and [Mn6] SMMs.12 Furthermore, the ligand pyridine-2-aldoximate has been utilized to construct several heterometallic polynuclear cluster complexes recently. For examples, Christou et al. assembled an unusual Ni8Dy8 ‘core–shell’ complex using the pyridine-2-aldoxime directly;13 while the Miyasaka group and the Chen group employed ‘metal oximate’ [Ni(pao)2(py)2] and [Ni(paoH)2Cl2] as the starting materials to construct several heterometallic polynuclear Ni–Mn clusters, respectively.14 On the other hand, the starting materials Ln(acac)2(CH3COO)(H2O)2 have been explored to construct the cluster complexes with three types of organic ligands, which include the bridging acetate ligand, the chelating hfac− ligand and the second bridging ligand.9 In order to obtain 1 and 2 in a simple way, we also adopted the strategy of using the ‘metal oximates’ as ligands, and Ln(acac)2(CH3COO)(H2O)2 were used as another starting materials. When Ni(paoH)2Cl2 was treated with Ln(acac)2(CH3COO)(H2O)2 in methanol in the presence of Et3N, 1 and 2 could be produced easily. Obviously, Et3N provided the basic conditions to facilitate deprotonation of the oxime. The preparation of 1 and 2 can be represented by eqn (1).| 3Ni(paoH)2Cl2 + 4Ln(acac)2(CH3COO)(H2O)2 + 6Et3N + 2MeOH = [Ni2Ln4(pao)6(acac)8(CH3COO)2(MeOH)] ·H2O·MeOH + Ni(CH3COO)2 + 6Et3NHCl + 7H2O | (1) |
The two ‘one-pot’ reactions are simple, but yields good.
Crystal structures
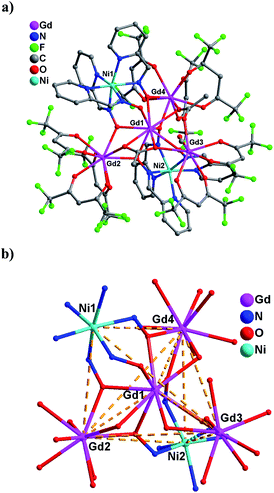
Both complexes crystallize in the space group P21/c. As shown in Fig. 1, the hexanuclear metal core of 1 can be described as a distorted trigonal bipyramid but some edges are fictitious, in which the [Gd4] triangle plane possesses three Gd3+ ions at the corners and one central Gd3+ ion lying 0.5942 Å above the plane; while two Ni2+ ions occupy the apical sites. There are two types of peripheral Gd3+ ions according to the coordination modes: the Gd2 and Gd4 atoms have a nearly monocapped square-antiprism environment (Fig. S1†), completed by six oxygen atoms from three hfac− ligands, two oximate oxygen atoms from two Ni(pao)3− building blocks, and one oxygen atom from the η1:η2:μ3-AcO− ligand (for Gd4) or the η2:η2:μ4-AcO− ligand (for Gd2); whereas the Gd3 atom shows a distorted square-antiprism coordinate geometry(Fig. S1†), bonded by four oxygen atoms from two hfac− ligands, one oxygen atom from the η1:η2:μ3-AcO− ligand, one oxygen atom from the η2:η2:μ4-AcO− ligand, and one oxygen atom from the terminal methanol molecule. The centered Gd1 atom also exhibits a distorted monocapped square-antiprism configuration (Fig. S1†), but coordinated by six oximate oxygen atoms from two Ni(pao)3− building blocks, two oxygen atoms from the η2:η2:μ4-AcO− ligand, and one oxygen atom from the η1:η2:μ3-AcO− ligand. The Gd–O bond lengths of the eight-coordinate Gd3 atom (average value of 2.375 Å) are obviously smaller than those for the nine-coordinate Gd atoms (mean values of 2.477, 2.429 and 2.442 Å for the Gd1, Gd2 and Gd4 atoms, respectively).There are two crystallographically independent nickel atoms, each of which is bonded by three pao− ligands in a perpendicular way, exhibiting a distorted [NiN6] octahedron geometry. The Ni–N bond distances for the Ni1 atom (average 2.068 Å) are very close to those for the Ni2 atom (2.069 Å). Interestingly, all three oximate groups of the Ni(pao)3− building block are situated at the same side in order to bond the central Gd1 atom, acting as a tridentate ‘metal oximate’ ligand. Besides, three oximate oxygen atoms from three pao− ligands of the Ni1 atom further cement the [Gd4] triangle plane through bridging the three peripheral Gd3+ atoms to the central one, while the other Ni(pao)3− building block (with the Ni2 atom) connects only two terminal Gd3+ atoms (Gd2 and Gd4) with the centered Gd1 atom. The formed trigonal bipyramid topology is reminiscent of that of Mn{Mn(hfac)2}3{Ni(pao)3}2,14a where the Ni(pao)3− building blocks at two apical sites link to the [Mn4] triangle plane from the above and below two directions. Furthermore, there are strong hydrogen bonds between the solvent methanol molecule and the coordinated methanol molecule (O28⋯O27 = 2.608 Å) and between the solvent methanol molecule and the oximate oxygen atom O3 from the Ni(pao)3− building block with the Ni1 atom (O28⋯O3 = 2.621 Å), which are propitious to stabilize the whole crystal structure.
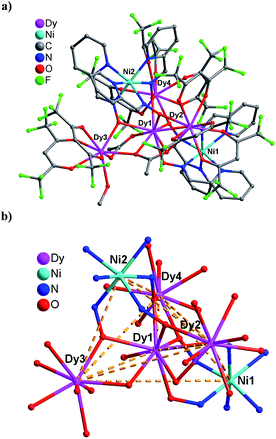
Interestingly, although the cell parameters of complex 2 is quite similar to those of 1, and both complexes possess the same trigonal bipyramid metal skeleton, there are some subtle structure differences between 1 and 2 worthy to be mentioned. As shown in Fig. 2, the centered Dy1 atom in the [Dy4] triangle plane of 2 exhibits the same monocapped square-antiprism configuration as the Gd1 atom in 1 (Fig. S2†), however, there are three types of peripheral Dy3+ ions based on the coordination modes: the Dy2 atom has a monocapped square-antiprism environment similar to that of the Gd2 atom in 1 (Fig. S2†), and the Dy3 atom displays a distorted square-antiprism coordinate geometry corresponding to that for the Gd3 atom in 1 (Fig. S2†); while the Dy4 atom is eight-coordinate rather than nine-coordinate (Fig. S2†), bonded by six oxygen atoms from three hfac− ligands, and two oximate oxygen atoms from two Ni(pao)3− building blocks, exhibiting a heavily distorted square-antiprism environment, which is obviously different from the monocapped square-antiprism environment of the Gd4 atom in 1. In addition, both complexes have the η2:η2:μ4-AcO− ligand bridging the Ln2, Ln1 and Ln3 atoms (Ln = Gd and Dy for 1 and 2, respectively), however, complex 1 possesses the η1:η2:μ3-AcO− ligand that connects with the Gd4, Gd1 and Gd3 atoms, whereas the corresponding AcO− ligand in complex 2 only links to the Dy1 and Dy2 atoms using the η1:η1:μ2-bridging mode.
The coordinating and bridging modes of the Ni(pao)3− building blocks in 2 are the same as those in 1, which make these two compounds to have the same trigonal bipyramid metal topology. Furthermore, similar strong hydrogen bonds (O28⋯O27 = 2.646 Å and O28⋯O3 = 2.639 Å) are observed for 2. The Dy–O bond distances of the eight-coordinate Dy atoms (average value of 2.364 and 2.366 Å for the Dy3 and Dy4 atoms, respectively) are also obviously smaller than those for the nine-coordinate Dy atoms (mean values of 2.445 and 2.410 Å for the Dy1 and Dy2 atoms, respectively). It is noteworthy that the Dy–O bond lengths of 2 are a little smaller than the corresponding Gd–O bond distances in 1, as expected, which is ascribed to the radius contraction from the Gd3+ ion to the Dy3+ ion.
Magnetic properties
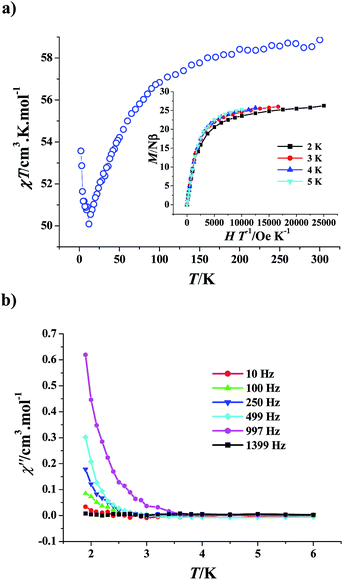
The magnetic properties of 1 were investigated as a function of field and temperature. As shown in Fig. 3a, the χT value at room temperature is 34.26 cm3 K mol−1, slightly larger than that expected value (33.5 cm3 K mol−1) for two Ni2+ (S = 1) and four Gd3+ (S = 7/2, L = 0, 8S7/2) non-coupling ions (assuming gGd = gNi = 2.0). The χT product increases gradually as the temperature is reduced, then rises sharply when the temperature is lower than 25 K, and finally reaches the largest value of 45.20 cm3 K mol−1 at 2 K, suggesting weak ferromagnetic interactions among the metal ions. The magnetic susceptibility data were analyzed by the Curie–Weiss law, extracting the Curie constants (C) of 34.21 cm3 K mol−1 and the Weiss constants (Θ) of 1.31 K. The small positive Θ value validates the weak ferromagnetic interaction.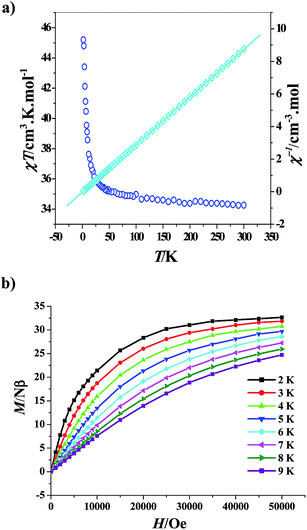 | ||
| Fig. 3 Plots of χT versus T (○) and 1/χ versus T (◊) of complex 1, the solid line represents the best theoretical fitting (a). Isothermal field-dependent curves in the range T = 2–9 K for 1 (b). | ||
It is well known that the ferromagnetic coupling among the metal ions brings to a large spin ground state S, which may induce the large magnetic entropy change {−ΔSm = R![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(2S + 1)}15 and the corresponding large MCE. Therefore, the field (H) dependence of the magnetizations (M) of 1 was measured at low temperatures (2–9 K) (Fig. 3b). Under the 50 kOe magnetic field, the magnetization (32.66 Nβ) at 2 K is slightly larger than the saturation value of 32 Nβ expected for an S = 16 system (g = 2), further confirming the ferromagnetic interaction. Furthermore, the magnetization slowly increases to reach the saturation value as the field enhances, which indicates that the population of low-lying levels possesses a small magnetic moment only becomes depopulated after employing a large field.4f To examine the magnetic anisotropy, the magnetization (M/Nβ) versus H/T curves were reduced, in which the isofield lines are almost superimposed (Fig. 4a), suggesting the weak magnetic anisotropy, which favors the MCE.
ln(2S + 1)}15 and the corresponding large MCE. Therefore, the field (H) dependence of the magnetizations (M) of 1 was measured at low temperatures (2–9 K) (Fig. 3b). Under the 50 kOe magnetic field, the magnetization (32.66 Nβ) at 2 K is slightly larger than the saturation value of 32 Nβ expected for an S = 16 system (g = 2), further confirming the ferromagnetic interaction. Furthermore, the magnetization slowly increases to reach the saturation value as the field enhances, which indicates that the population of low-lying levels possesses a small magnetic moment only becomes depopulated after employing a large field.4f To examine the magnetic anisotropy, the magnetization (M/Nβ) versus H/T curves were reduced, in which the isofield lines are almost superimposed (Fig. 4a), suggesting the weak magnetic anisotropy, which favors the MCE.
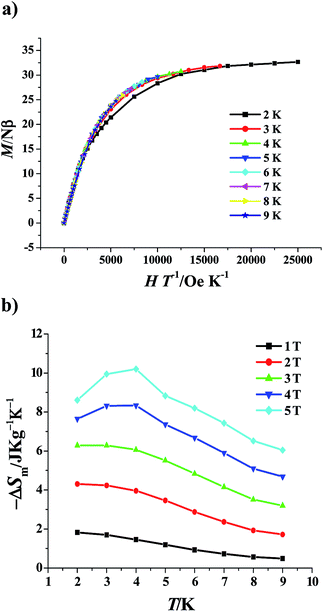 | ||
| Fig. 4 Plots of reduced magnetization (M) versus H/T for 1 (a). Magnetic entropy change (−ΔSm) versus T of 1 for applied field changes ΔH (b). | ||
The Maxwell relation ΔSm(T)ΔH = ∫[∂M(T,H)/∂T]HdH was adopted to calculate the magnetic entropy changes −ΔSm.16 The results are shown in Fig. 4b in the −ΔSm versus T curve formation. Obviously, the stronger is the ΔH applied, the larger is the −ΔSm value obtained. Notably, the largest −ΔSm value for the accessible maximum ΔH of 50 kOe at 4 K is 10.2 J kg−1 K−1, which is clearly larger than the theoretical maximum entropy for S = 16, i.e. R![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(2S + 1) = 3.50R = 29.07 J mol−1 K−1 = 8.73 J kg−1 K−1. This means the MCE is enhanced, which maybe originates from the presence of low-lying excited S states thermally accessible even at very low temperatures, such excited states result in an excess of magnetic entropy.4a,c,e,f To the best of our knowledge, only limited manganese, iron and Mn–Gd cluster complexes have been observed to show the enhanced MCE by the Brechin, Dalgarno, Evangelisti, and McInnes groups.4a,c,e,f Although many Ni–Ln heterometallic clusters have been reported to exhibit the MCE,5 their largest −ΔSm values at 50 kOe are all smaller than or close to the theoretical value calculated by the equation R
ln(2S + 1) = 3.50R = 29.07 J mol−1 K−1 = 8.73 J kg−1 K−1. This means the MCE is enhanced, which maybe originates from the presence of low-lying excited S states thermally accessible even at very low temperatures, such excited states result in an excess of magnetic entropy.4a,c,e,f To the best of our knowledge, only limited manganese, iron and Mn–Gd cluster complexes have been observed to show the enhanced MCE by the Brechin, Dalgarno, Evangelisti, and McInnes groups.4a,c,e,f Although many Ni–Ln heterometallic clusters have been reported to exhibit the MCE,5 their largest −ΔSm values at 50 kOe are all smaller than or close to the theoretical value calculated by the equation R![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(2S + 1), so complex 1 is the first Ni–Ln cluster complex with the remarkably enhanced MCE. Furthermore, the maximum entropy of 1 is larger than the highest values of some gadolinium-containing intermetallic compounds, for instances, Gd7Pd3 (ref. 17a) and Gd12Co7,17b complex 1 is thus a good candidate for the cryogenic magnetic refrigeration materials.
ln(2S + 1), so complex 1 is the first Ni–Ln cluster complex with the remarkably enhanced MCE. Furthermore, the maximum entropy of 1 is larger than the highest values of some gadolinium-containing intermetallic compounds, for instances, Gd7Pd3 (ref. 17a) and Gd12Co7,17b complex 1 is thus a good candidate for the cryogenic magnetic refrigeration materials.
The magnetic behaviours of 2 seem to be quite different from those of 1. As depicted in Fig. 5a, the room temperature χT value of 58.86 cm3 K mol−1 accords with the theory value of 58.68 cm3 K mol−1 expected for a non-interacting Ni2Dy4 unit (assuming gNi = 2.0). It decreases continuously with lowering temperature, reaching a minimum value of 50.09 cm3 K mol−1 at 12 K, mainly due to the crystal-field effects (thermal depopulation of the Dy3+ Stark sublevels). Below this temperature, the χT product abruptly increases to reach 53.57 cm3 K mol−1 at 2 K, suggesting the existence of ferromagnetic interactions among the metal ions, as seen for 1. Therefore, it is not always credible to judge the nature of the magnetic exchange interaction just according to the temperature dependence of the χT product for the Dy(III)-based complexes. The comparison of the magnetic interactions between 1 and 2 may ascertain the ferromagnetic interactions for 2 at last. Such a method is feasible and has been adopted by several groups to assume the ferromagnetic interactions between the 3d metal ion (Ni2+ and Co2+) and the lanthanide ion (Tb3+, Dy3+ and Ho3+).5i,18
The field dependence of the magnetization at 2–5 K revealed a rapid increase of magnetization at low magnetic fields, which is in agreement with a high-spin state of ferromagnetic interactions. However, no any sign of saturation could be reached owing to the crystal-field splitting of the ground multiplet of the Dy3+ ion. The M versus H/T curves are not superimposed (Fig. 5a, inset), suggesting the significant magnetic anisotropy and/or low-lying excited states, required for a SMM. Therefore, alternating-current (ac) magnetic susceptibility measurements were carried out under a 2.5 Oe oscillating field to investigate the dynamics of magnetization. When the dc field was zero, the out-of-phase signals of complex 2 are frequency-dependent, indicating slow relaxation of the magnetization (Fig. 5b). However, net maxima of the χ′′ signals were not observed even at frequencies as high as 1400 Hz. One possible reason is the existence of fast quantum tunneling effects. Nevertheless, after applying a dc field of 2000 Oe to fully or partly suppress the quantum tunneling effects, the χ′′ signals are still not significantly improved, indicating that quenching of the quantum tunneling effects by this dc field can be negligible. Such a phenomenon was also observed in the other Dy-containing SMMs before.5i,19 In addition, the M versus H plot measured at 1.9 K does not show any hysteresis (Fig. S3†).
Conclusions
In summary, this work describes two novel ferromagnetic hexanuclear 3d–4f cluster complexes. They have the same ‘trigonal bipyramid’ metal topology, but one peripheral Ln3+ ion of them shows subtle differences on the coordination geometry. Two types of magnetic properties were observed for them, mainly dependent on the lanthanide ions. The [Ni2Gd4] cluster shows an enhanced MCE mainly due to an excess of entropy change supplied by the low-lying excited S states. Such an enhanced MCE is a rare observation for the cluster complexes. On the other hand, the [Ni2Dy4] complex displays slow magnetic relaxation of the SMM mainly owing to the large magnetic anisotropy of the Dy3+ ion.Acknowledgements
This work was supported by National Key Basic Research Program of China (2013CB933403), National Natural Science Foundation of China (21073198 and 91022014), and the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB12010103).Notes and references
- C. Benelli and D. Gatteschi, Chem. Rev., 2002, 102, 2369 CrossRef CAS PubMed.
- Y.-Z. Zheng, G.-J. Zhou, Z. Zheng and R. E. P. Winpenny, Chem. Soc. Rev., 2014, 43, 1462 RSC.
- (a) R. Sessoli, D. Gatteschi, A. Caneschi and M. A. Novak, Nature, 1993, 365, 141 CrossRef CAS; (b) G. Christou, D. Gatteschi, D. N. Hendrickson and R. Sessoli, MRS Bull., 2000, 25, 66 CrossRef CAS; (c) D. Gatteschi and R. Sessoli, Angew. Chem., Int. Ed., 2003, 42, 268 CrossRef CAS PubMed; (d) G. Aromi and E. K. Brechin, Struct. Bonding, 2006, 122, 1 CrossRef CAS; (e) L. M. C. Beltran and J. R. Long, Acc. Chem. Res., 2005, 38, 325 CrossRef CAS PubMed; (f) R. Bagai and G. Christou, Chem. Soc. Rev., 2009, 38, 1011 RSC; (g) V. Chandrasekhar and B. Murugesapandian, Acc. Chem. Res., 2009, 42, 1047 CrossRef CAS PubMed; (h) R. Sessoli and A. K. Powell, Coord. Chem. Rev., 2009, 253, 2328 CrossRef CAS PubMed; (i) G. E. Kostakis, A. M. Akoab and A. K. Powell, Chem. Soc. Rev., 2010, 39, 2238 RSC; (j) M. Murrie, Chem. Soc. Rev., 2010, 39, 1986 RSC; (k) D. N. Woodruff, R. E. P. Winpenny and R. A. Layfield, Chem. Rev., 2013, 113, 5110 CrossRef CAS PubMed; (l) B.-W. Wang, X.-Y. Wang, H.-L. Sun, S.-D. Jiang and S. Gao, Philos. Trans. R. Soc., A, 2013, 371, 20120316 CrossRef PubMed; (m) T. Komeda, H. Isshiki, J. Liu, Y.-F. Zhang, N. Lorente, K. Katoh, B. K. Breedlove and M. Yamashita, Nat. Commun., 2011, 2, 217 CrossRef PubMed; (n) R. Vincent, S. Klyatskaya, M. Ruben, W. Wernsdorfer and F. Balestro, Nature, 2012, 488, 357 CrossRef CAS PubMed.
- (a) M. Evangelisti, A. Candini, A. Ghirri, M. Affronte, E. K. Brechin and E. J. L. McInnes, Appl. Phys. Lett., 2005, 87, 072504 CrossRef PubMed; (b) M. Evangelisti, F. Luis, L. J. de Jongh and M. Affronte, J. Mater. Chem., 2006, 16, 2534 RSC; (c) R. Shaw, R. H. Laye, L. F. Jones, D. M. Low, C. Talbot-Eeckelaers, Q. Wei, C. J. Milios, S. Teat, M. Helliwell, J. Raftery, M. Evangelisti, M. Affronte, D. Collison, E. K. Brechin and E. J. L. McInnes, Inorg. Chem., 2007, 46, 4968 CrossRef CAS PubMed; (d) M. Manoli, R. D. L. Johnstone, S. Parsons, M. Murrie, M. Affronte, M. Evangelisti and E. K. Brechin, Angew. Chem., Int. Ed., 2007, 46, 4456 CrossRef CAS PubMed; (e) M. Manoli, A. Collins, S. Parsons, A. Candini, M. Evangelisti and E. K. Brechin, J. Am. Chem. Soc., 2008, 130, 11129 CrossRef CAS PubMed; (f) G. Karotsis, M. Evangelisti, S. J. Dalgarno and E. K. Brechin, Angew. Chem., Int. Ed., 2009, 48, 9928 CrossRef CAS PubMed; (g) S. K. Langley, N. F. Chilton, B. Moubaraki, T. Hooper, E. K. Brechin, M. Evangelisti and K. S. Murray, Chem. Sci., 2011, 2, 1166 RSC; (h) M. Evangelisti, O. Roubeau, E. Palacios, A. Camón, T. N. Hooper, E. K. Brechin and J. J. Alonso, Angew. Chem., Int. Ed., 2011, 50, 6606 CrossRef CAS PubMed; (i) J.-D. Leng, J.-L. Liu and M.-L. Tong, Chem. Commun., 2012, 48, 5286 RSC; (j) Z.-M. Zhang, L.-Y. Pan, W.-Q. Lin, J.-D. Leng, F.-S. Guo, Y.-C. Chen, J.-L. Liu and M.-L. Tong, Chem. Commun., 2013, 49, 8081 RSC; (k) Y.-L. Hou, G. Xiong, P.-F. Shi, R.-R. Cheng, J.-Z. Cui and B. Zhao, Chem. Commun., 2013, 49, 6066 RSC; (l) J.-P. Zhao, R. Zhao, Q. Yang, B.-W. Hu, F.-C. Liu and X.-H. Bu, Dalton Trans., 2013, 42, 14509 RSC; (m) G. Xiong, H. Xu, J.-Z. Cui, Q.-L. Wang and B. Zhao, Dalton Trans., 2014, 43, 5639 RSC; (n) K. S. Pedersen, G. Lorusso, J. J. Morales, T. Weyhermüller, S. Piligkos, S. K. Singh, D. Larsen, M. Schau-Magnussen, G. Rajaraman, M. Evangelisti and J. Bendix, Angew. Chem., Int. Ed., 2014, 53, 2394 CrossRef CAS PubMed; (o) I. Kornarakis, G. Sopasis, C. J. Milios and G. S. Armatas, RSC Adv., 2012, 2, 9809 RSC.
- (a) Y. Z. Zheng, M. Evangelisti and R. E. Winpenny, Angew. Chem., Int. Ed., 2011, 50, 3692 CrossRef CAS PubMed; (b) J. B. Peng, Q. C. Zhang, X. J. Kong, Y. P. Ren, L. S. Long, R. B. Huang, L. S. Zheng and Z. Zheng, Angew. Chem., Int. Ed., 2011, 50, 10649 CrossRef CAS PubMed; (c) J. B. Peng, Q. C. Zhang, X. J. Kong, Y. Z. Zheng, Y. P. Ren, L. S. Long, R. B. Huang, L. S. Zheng and Z. Zheng, J. Am. Chem. Soc., 2012, 134, 3314 CrossRef CAS PubMed; (d) T. N. Hooper, J. Schnack, S. Piligkos, M. Evangelisti and E. K. Brechin, Angew. Chem., Int. Ed., 2012, 51, 4633 CrossRef CAS PubMed; (e) Z. Y. Li, J. Zhu, X. Q. Wang, J. Ni, J. J. Zhang, S. Q. Liu and C. Y. Duan, Dalton Trans., 2013, 42, 5711 RSC; (f) J. W. Sharples and D. Collison, Polyhedron, 2013, 54, 91 CrossRef CAS PubMed; (g) P. Wang, S. Shannigrahi, N. L. Yakovlev and T. S. Andy Hor, Dalton Trans., 2014, 43, 182 RSC; (h) T. D. Pasatoiu, A. Ghirri, A. M. Madalan, M. Affronte and M. Andruh, Dalton Trans., 2014, 43, 9136 RSC; (i) S. Das, A. Dey, S. Kundu, S. Biswas, A. J. Mota, E. Colacio and V. Chandrasekhar, Chem.–Asian J., 2014, 7, 1876 CrossRef PubMed; (j) A. Upadhyay, N. Komatireddy, A. Ghirri, F. Tuna, S. K. Langley, A. K. Srivastava, E. C. Sañudo, B. Moubaraki, K. S. Murray, E. J. L. McInnes, M. Affronte and M. Shanmugam, Dalton Trans., 2014, 43, 259 CAS.
- (a) C.-M. Liu, D.-Q. Zhang, X. Hao and D.-B. Zhu, Cryst. Growth Des., 2012, 12, 2948 CrossRef CAS; (b) C.-M. Liu, D.-Q. Zhang and D. B. Zhu, Inorg. Chem., 2013, 52, 8933 CrossRef CAS PubMed; (c) C.-M. Liu, D.-Q. Zhang and D. B. Zhu, Dalton Trans., 2013, 42, 14813 RSC; (d) C.-M. Liu, D.-Q. Zhang, X. Hao and D.-B. Zhu, Chem.–Asian J., 2014, 7, 1841 Search PubMed; (e) C.-M. Liu, D.-Q. Zhang and D. B. Zhu, RSC Adv., 2014, 4, 36053 RSC.
- C.-M. Liu, D.-Q. Zhang and D. B. Zhu, Dalton Trans., 2010, 39, 11325 RSC.
- R. A. Krause and D. H. Busch, J. Am. Chem. Soc., 1960, 82, 4830 CrossRef CAS.
- (a) H.-B. Xu, J. Li, L.-Y. Zhang, X. Huang, B. Li and Z.-N. Chen, Cryst. Growth Des., 2010, 10, 4101 CrossRef CAS; (b) H.-B. Xu, Y.-T. Zhong, W.-X. Zhang, Z.-N. Chen and X.-M. Chen, Dalton Trans., 2010, 39, 5676 RSC.
- O. Kahn, Molecular Magnetism, VCH, New York, 1993 Search PubMed.
- (a) G. M. Sheldrick, SHELXS 97, Program for the Solution of Crystal Structures, University of Göttingen, Göttingen, Germany, 1997 Search PubMed; (b) G. M. Sheldrick, SHELXL 97, Program for the Refinement of Crystal Structures, University of Göttingen, Göttingen, Germany, 1997 Search PubMed.
- For examples: (a) C. J. Milios, A. Vinslava, P. A. Wood, S. Parsons, W. Wernsdorfer, G. Christou, S. P. Perlepes and E. K. Brechin, J. Am. Chem. Soc., 2007, 129, 8 CrossRef CAS PubMed; (b) C.-I. Yang, W. Wernsdorfer, G.-H. Lee and H.-L. Tsai, J. Am. Chem. Soc., 2007, 129, 456 CrossRef CAS PubMed; (c) C. J. Milios, A. Vinslava, S. Moggach, S. Parsons, W. Wernsdorfer, G. Christou, S. P. Perlepes and E. K. Brechin, J. Am. Chem. Soc., 2007, 129, 2754 CrossRef CAS PubMed; (d) C. J. Milios, R. Inglis, A. Vinslava, R. Bagai, W. Wernsdorfer, S. Parsons, S. P. Perlepes, G. Christou and E. K. Brechin, J. Am. Chem. Soc., 2007, 129, 12505 CrossRef CAS PubMed; (e) C. J. Milios, R. Inglis, R. Bagai, W. Wernsdorfer, A. Collins, S. Moggach, S. Parsons, S. P. Perlepes, G. Christou and E. K. Brechin, Chem. Commun., 2007, 3476 RSC; (f) C. C. Stoumpos, R. Inglis, O. Roubeau, H. Sartzi, A. A. Kitos, C. J. Milios, G. Aromí, A. J. Tasiopoulos, V. Nastopoulos, E. K. Brechin and S. P. Perlepes, Inorg. Chem., 2010, 49, 4388 CrossRef CAS PubMed.
- C. Papatriantafyllopoulou, T. C. Stamatatos, C. G. Efthymiou, L. Cunha-Silva, F. A. A. Paz, S. P. Perlepes and G. Christou, Inorg. Chem., 2010, 49, 9743 CrossRef CAS PubMed.
- (a) H. Miyasaka, T. Nezu, F. Iwahori, S. Furukawa, K. Sugimoto, R. Clérac, K.-I. Sugiura and M. Yamashita, Inorg. Chem., 2003, 42, 4501 CrossRef CAS PubMed; (b) H. Chen, C.-B. Ma, D.-Q. Yuan, M.-Q. Hu, H.-M. Wen, Q.-T. Liu and C.-N. Chen, Inorg. Chem., 2011, 50, 10342 CrossRef CAS PubMed.
- M. Evangelisti and E. K. Brechin, Dalton Trans., 2010, 39, 4672 RSC.
- V. K. Pecharsky and K. A. Gschneidner Jr, J. Magn. Magn. Mater., 1999, 200, 44 CrossRef CAS.
- (a) F. Canepa, M. Napoletano and S. Cirafici, Intermetallics, 2002, 10, 731 CrossRef CAS; (b) X. Chen and Y. H. Zhuang, Solid State Commun., 2008, 148, 322 CrossRef CAS PubMed.
- (a) V. Chandrasekhar, B. M. Pandian, R. Azhakar, J. J. Vittal and R. Clérac, Inorg. Chem., 2007, 46, 5140 CrossRef CAS PubMed; (b) V. Chandrasekhar, B. M. Pandian, J. J. Vittal and R. Clérac, Inorg. Chem., 2009, 48, 1148 CrossRef CAS PubMed; (c) T. Yamaguchi, J. P. Costes, Y. Kishima, M. Kojima, Y. Sunatsuki, N. Brefuel, J. P. Tuchagues, L. Vendier and W. Wernsdorfer, Inorg. Chem., 2010, 49, 9125 CrossRef CAS PubMed; (d) E. Colacio, J. Ruiz, A. J. Mota, M. A. Palacios, E. Cremades, E. Ruiz, F. J. White and E. K. Brechin, Inorg. Chem., 2012, 51, 5857 CrossRef CAS PubMed; (e) M. Towatari, K. Nishi, T. Fujinami, N. Matsumoto, Y. Sunatsuki, M. Kojima, N. Mochida, T. Ishida, N. Re and J. Mrozinski, Inorg. Chem., 2013, 52, 6160 CrossRef CAS PubMed.
- S. Titos-Padilla, J. Ruiz, J. M. Herrera, E. K. Brechin, W. Wersndorfer, F. Lloret and E. Colacio, Inorg. Chem., 2013, 52, 9620 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Additional figures of the crystal structure (Fig. S1 and S2) and magnetic characterization (Fig. S3). CCDC 1016867 and 1016868. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra07882a |
| This journal is © The Royal Society of Chemistry 2014 |