SERS performance of graphene oxide decorated silver nanoparticle/titania nanotube array
Abstract
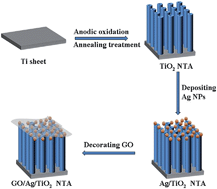
A graphene oxide (GO) decorated silver nanoparticle/titania nanotube array (Ag/TiO2 NTA) has been designed as a surface-enhanced Raman scattering (SERS) substrate for the sensitive detection of organic molecules. Anatase TiO2 NTA was synthesized by controlled anodic oxidation and annealing treatment processes. Ag/TiO2 NTA was formed by depositing silver nanoparticles (Ag NPs) on the surface of anatase TiO2 NTA through the polyol process. GO/Ag/TiO2 NTA was prepared by decorating GO on the surface of Ag/TiO2 NTA through an impregnation process. Methyl blue (MB) was used as the probe molecule to evaluate the adsorption capability and SERS activity of the as-prepared SERS substrates. The adsorption ratio of MB was increased from 25.2% for Ag/TiO2 NTA up to 38.0% for GO/Ag/TiO2 NTA, presenting an obvious improvement of the adsorption capability. The analytical enhancement factor was increased from 1.06 × 104 for Ag/TiO2 NTA up to 3.67 × 104 for GO/Ag/TiO2 NTA, presenting an obvious improvement of SERS detection performance. The GO/Ag/TiO2 NTA substrate accordingly achieved a very low detection limit of 1.0 × 10−9 M MB. GO/Ag/TiO2 NTA also exhibited good adsorption capability to bisphenol A (BPA) with a weak affinity, presenting a SERS detection limit of 5 × 10−7 M BPA. Moreover, GO/Ag/TiO2 NTA was able to achieve self-cleaning functionality through photocatalytic degradation of organic molecules adsorbed on the SERS substrate under solar light irradiation. GO/Ag/TiO2 NTA having good adsorption capability, self-cleaning properties, reproducibility and cycleability contributed to a promising application for sensitive and recyclable SERS detection.

 Please wait while we load your content...
Please wait while we load your content...