Flexible superhydrophobic paper with a large and stable floating capacity†
Jihua Zhang *,
Huadong Feng,
Weitao Zao,
Mingbo Ling and
Yunfeng Zhao
*,
Huadong Feng,
Weitao Zao,
Mingbo Ling and
Yunfeng Zhao
Aerospace Research Institute of Material and Processing Technology, Beijing, 100076, P. R. China. E-mail: zjhicca@iccas.ac.cn; Fax: +86 01068382974; Tel: +86 01068383313
First published on 9th September 2014
Abstract
Recently, one interesting wetting phenomenon was found whereby a live frog can stably stand or easily jump on a floating lotus surface. Inspired by the floating leaf, constructing a lotus-like substrate is important to provide powerful aquatic support. In this article, a flexible and light-weight superhydrophobic paper was successfully fabricated by coating a mixture of polystyrene-poly(methyl methacrylate) copolymer (PS-co-PMMA) and silica nano-particles. The micro/nano structures from the composite coating render common papers to possess superhydrophobic surfaces with low adhesion. The coated paper restricts capillary absorption owing to its high water-repellency; moreover, it has an ultra-low absorption when completely soaked into water. A lotus-like support model with some origami frogs is made to display the floating feasibility of the coated paper. The maximum supporting force of the coated paper with an area of 30 × 30 mm is equivalent to ∼68 times its own weight. The simple force analysis illustrates that the great carrying capacity of the coated paper comes from its superhydrophobicity. Importantly, the flexibility of the coated paper possibly gives rise to its good dynamic floating stability. We believe that flexible superhydrophobic paper can be practically applied to some smart designs like aquatic micro-devices.
1. Introduction
Recently, the striking loading capacity of superhydrophobic legs of water striders has attracted considerable attention.1–3 The insect's legs are composed of a lot of submicrometer-sized hair with fine nanogrooves, allowing its body to rush and even jump across water surface at high speeds.3 Inspired by the insect, some researchers have tried to design and fabricate novel drag-reducing and fast propulsion aquatic or air devices. For example, superhydrophobic wires, round-shaped copper foil or mesh boats in the size range of a few centimeters were made as artificial “legs” to provide sufficient load supports.4–8 With the aids of these “legs”, the micro-devices can freely move and jump on the water surface just as a water strider does.9–11 Although no one doubts the achievements in the field, there are still some basic questions that remain unclear. For instance, what can be done to improve the stability of these floating micro-devices? How can we resolve their duration when they float for a long time? Therefore, it is very important to study the stable and durable superhydrophobic substrates to serve aquatic applications.Prior to water striders' legs, the superhydrophobicity of lotus leaf has been discovered earlier by researchers.12–18 Lots of experiments have shown that the top, bottom surface and rim of this leaf possess many amazing wetting behaviors,19 but there are a few concerns about the leaf's floating on the water surface although it belongs to an aquatic plant. Lately, another interesting phenomenon about the floating leaf has captured our attention. Fig. 1 shows that a live frog can stand on a floating leaf surface with a diameter of ∼50 cm and not sink. Moreover, such a frog is able to easily jump from one lotus leaf to another. This suggests a great load support from the flexible superhydrophobic leaf surface. What is most appealing is that the frog can stably stay on its surface, even when the lotus leaf is partly submerged. The amazing floating behavior of the lotus leaf hints that flexible lotus-like substrates may take advantage of providing stable floating abilities. Moreover, fabricating such lotus-like substrates can bring more practical applications for aquatic devices than those artificial “insect legs” due to their larger supporting force.
Up to now, many approaches, such as lithographic patterning, laser/plasma etching, vertical alignment of nanotubes/nanofibers, sol–gel methods, phase separation, assembly, glancing angle deposition, and solution-immersion methods have been developed to prepare superhydrophobic surfaces.20–33 Among them, the solution-immersion method has been found to be a simple and effective technique for depositing large area superhydrophobic coatings onto flexible substrates.34 What is more, it gives rise to advantages such as uniform deposits on the objects with desired shapes, and short processing times. Additionally, flexible substrates including elastomer, polymer film, paper, fabric and spongia are available in our daily life. It is naturally considered that the flexible substrates should maintain as little weight as possible to maximize their supporting forces on water. Paper and spongia are therefore preferred due to their low densities from their porous structures. In contrast with ultra soft spongia, it can be easier to produce a thin film from paper, as well as inexpensive, biodegradable and renewable, and thus paper is a promising candidate as a flexible superhydrophobic substrate.33 However, common paper strongly absorbs water. A water drop can completely spread on common paper within 20 s.33,35,36 This brings possible duration concerns for artificial superhydrophobic paper when it floats for a long time. Therefore, it becomes a challenge to fabricate a flexible and durable superhydrophobic paper with the capacity to stably float.
In this study, we prepared superhydrophobic coatings on common print paper by using solution-immersion methods. Generally, hydrophobic polymers, such as polystyrene, can be used to prepare superhydrophobic paper by solution-immersion methods.29 Here, we synthesized a block copolymer of polystyrene-poly(methyl methacrylate) (PS-co-PMMA) for coating onto common paper due to its good consistency for surface roughness on common paper. To ensure that the coated paper floats, its water absorption was measured. After that, a lotus-like support model was constructed to practically display the floating feasibility of coated paper for loading micro-devices. Finally, the origin of its stable supporting ability was illustrated by theoretical and experimental analysis. These findings can provide a novel strategy to make some stable floating supports that can freely move on water to carry micro-devices.
2. Materials and methods
2.1 Sample preparation
PS-co-PMMA block copolymer powder (Mw = 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 166 g mol−1; Mw(PS) = 21
166 g mol−1; Mw(PS) = 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 511 g mol−1, Mw(PMMA) = 13
511 g mol−1, Mw(PMMA) = 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 420 g mol−1 and polydispersity index = 1.06) was firstly prepared by anionic polymerization according to the literature.37 The white powder was dissolved in N,N-dimethyl formamide (DMF) to produce different concentration solutions. The common print or filter paper was steeped in the solution for approximately 10 s, removed, and naturally dried out at room temperature. To vary the wetting adhesions of the paper, hydrophobic silica nanoparticles (average particle size of ∼16 nm, Evonik Degussa) were ultrasonically dispersed for 20 min in the 10 mg ml−1 PS-co-PMMA solution. After that, the same immersion and drying operations were used on the common papers.
420 g mol−1 and polydispersity index = 1.06) was firstly prepared by anionic polymerization according to the literature.37 The white powder was dissolved in N,N-dimethyl formamide (DMF) to produce different concentration solutions. The common print or filter paper was steeped in the solution for approximately 10 s, removed, and naturally dried out at room temperature. To vary the wetting adhesions of the paper, hydrophobic silica nanoparticles (average particle size of ∼16 nm, Evonik Degussa) were ultrasonically dispersed for 20 min in the 10 mg ml−1 PS-co-PMMA solution. After that, the same immersion and drying operations were used on the common papers.
2.2 Instruments and observation
The common and coated papers' immersion absorption were systemically tested by soaking them in water for different times (the total time is 10 min). Prior to soaking, the paper was vacuum dried at 50 °C for 2 h. To avoid the floating effects of coated paper affecting its absorption, its edges were clamped by a weight of ∼300 g, then they were together submerged into water. When the paper was pulled out from water, its surface was blown dry by N2 gas for 5 s to remove the extra water attached to the paper surface and then immediately weighed. Immersion absorption was calculated by the ratio of the weight increment to the initial weight of the paper.
3. Results and discussion
Fig. 2 shows the SEM images of uncoated (common) and PS-co-PMMA coated print paper surfaces. Common paper is composed of lots of smooth cellulose fibers. Three dimensional pore structures form through random arranged fibers. Moreover, the cross-section of fibers is not circular but flattened (Fig. S1†). In contrast, after common papers are immersed and then dried, the PS-co-PMMA coating uniformly covers every fiber (Fig. 2b). Owing to the incompatibility of chain segments between PS and PMMA,38 rough polymeric nano-particles are shown on the paper fibers (Fig. 2c). The AFM scan in Fig. 2d further displays that the heights of the small nano-particles are limited below 40 nm. It suggests that these rough microstructures may affect the wettability of the coated paper surface.Fig. 3a shows the CA values of a 4 μl drop on the common and coated print paper by coating using various concentrations of PS-co-PMMA solution. Note that the common paper is superhydrophilic (CA is close to zero, for a plan view see Fig. 3b) and smooth and flat PS-co-PMMA is also hydrophilic (instinct CA of approximately 79°). With the increase in concentration, the CA values on coated paper increase and then decrease. When the concentration is between 10 and 20 mg ml−1, CA values on the coated paper reach a maximum. The optical image of a 4 μl droplet deposited on the coated paper (from solution concentration of 10 mg ml−1) is shown in Fig. 3c. A water CA of 154.5 ± 2.1° is measured on its surface. Moreover, the coated paper is very flexible. It can be easily bent to a curved shape using two fingers (Fig. 3d). Even after being bent, the CA value (153.1 ± 1.3°) of coated paper barely changes, but water droplets on all coated paper surfaces display high adhesion states. To illustrate this, a simple experiment is conducted where a droplet is hung from the needle and placed in contact with the paper (Fig. 3e–i). After the droplet contacts the paper, we try to withdraw the needle and depart the droplet from the coated paper. The droplet is prolonged and then breaks from the needle. Finally, its bottom is firmly attached to the paper surface, implying its high adhesion state. All sliding angles (SAs) on these coated papers are beyond 90°. Even if we turn over them, a 4 μl droplet could not fall off. This means that a droplet on the coated paper is in the Wenzel state. Clearly, such superhydrophobic papers with high adhesions are not able to possess the lotus-like wettability, so it seems necessary to change the droplets to the Cassie regime on the paper surfaces.
To pursuit such a wetting transition, bioinformation from the lotus surface may be important so the leaf is checked by SEM. Fig. 4a shows the surface morphology of the lotus leaf with the water CA of 153.5 ± 4.1° and SA of ∼4°. Note that there are lots of nano-hairs covering every micro-papilla. In contrast, nano-sized PS-co-PMMA coating does not provide enough roughness to suspend a water droplet and develop an air cushion under its bottom, reflecting a Wenzel state. Theoretically, it has been predicted that an increase in the air fraction at the interface between a water drop and a solid surface could lead to a switch in the dominant wetting state from a Wenzel to a Cassie regime.39 Accordingly, we introduced silica nano-particles into the composite coating in order to improve the amount of micro-protrudes, which is believed to be helpful in trapping air layers at the interface. After silica particles and 10 mg ml−1 PS-co-PMMA solution (SiO2 nano-particles![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PS-co-PMMA = 1
PS-co-PMMA = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were mixed, the coated paper was prepared by immersion method. Its surface morphology is shown in Fig. 4b. Papillae-like agglomerations can be found on every paper fiber, that is, nano-sized particles cover the micro-sized agglomerations (Fig. 4c). Water CA on the coated paper is examined as 161.2 ± 1.3° and SA holds below 5°, verifying a Cassie state. The comparison is also made in Fig. 4e–i that after contacting the coated paper, water droplet is always hung at the tip of the needle, instead of adhering on the paper. This observation reflects an ultra-low adhesion between the droplet and coated paper (Fig. S2†). Besides, after being repeated bending for 10 times, the flexible coated paper still maintains a high water CA (∼160°) and low SA (below 5°). Therefore, a flexible lotus-like superhydrophobic paper surface is achieved by the simple solution-immersion method.
1) were mixed, the coated paper was prepared by immersion method. Its surface morphology is shown in Fig. 4b. Papillae-like agglomerations can be found on every paper fiber, that is, nano-sized particles cover the micro-sized agglomerations (Fig. 4c). Water CA on the coated paper is examined as 161.2 ± 1.3° and SA holds below 5°, verifying a Cassie state. The comparison is also made in Fig. 4e–i that after contacting the coated paper, water droplet is always hung at the tip of the needle, instead of adhering on the paper. This observation reflects an ultra-low adhesion between the droplet and coated paper (Fig. S2†). Besides, after being repeated bending for 10 times, the flexible coated paper still maintains a high water CA (∼160°) and low SA (below 5°). Therefore, a flexible lotus-like superhydrophobic paper surface is achieved by the simple solution-immersion method.
When common paper is placed on water, it will quickly get wet and then sink. We ascribe the floating disability to its strong capillary absorption from porous structures (Fig. 2a). So the water (not a droplet) absorption of lotus-like superhydrophobic paper must be checked prior to its floating on water. Two kinds of experiments are included: capillary absorption and immersion absorption. Between them, capillary absorption is able to give some information about immediate contacts of water with the paper, but the long-term interaction between water and the paper needs to be examined by complete immersion (i.e. immersion absorption).
Accompanying the optic observations of capillary absorption, the curves of capillary force versus position are simultaneously recorded (Fig. 5). In the experiments, the common print paper is firstly used as a comparison. Once contacted, water climbs along the common paper (leaving an obvious trace, the top inset in Fig. 5) and gives a downwards force (positive values in force-position curve). As the contact time prolongs, the capillary force increases and then keeps constant. However, the water trace does not climb parallel to the paper surface, which is attributed to the inconsistent microstructures in the paper (Fig. 2a). In contrast, no water climbs along the superhydrophobic paper, indicating its good water repellency. Especially, after the superhydrophobic paper further moves downwards, a big dimple develops between air, water and paper (the bottom inset in Fig. 5). Moreover, all values of capillary force are negative after the paper contacts water, reflecting that the capillary absorption cannot occur on its surface. Thus superhydrophobic paper hinders the capillary absorption, and improves its floating feasibility.
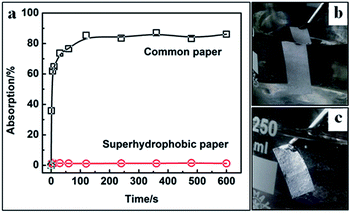 | ||
| Fig. 6 (a) Plots of water absorption versus soaking time of common and superhydrophobic paper; optical images of common (b) or superhydrophobic paper (c) when they are soaked in water. | ||
Fig. 6a shows the absorption of common and superhydrophobic paper in water, respectively. Superhydrophobic paper hardly takes any time to reach the equilibrium of absorption while common paper takes ∼120 s. Moreover, there is ultralow equilibrium absorption (close to zero) on the superhydrophobic paper, which is far less than that of common paper (∼84 wt%). It implies that the superhydrophobic paper cannot get wet even if it floats on the water for some time. Here, the short time of 10 min is chosen because the process of water absorption can be clearly seen. In fact, the low water absorption was maintained below 3 wt% for 30 hours. In additions, a sliver air reflection can be seen on superhydrophobic paper but it did not occur on the common paper (their optical images are shown in Fig. 6b and c, respectively). We believe that the low water absorption of superhydrophobic paper is helpful to ensuring its long-term floating ability.
 | ||
| Fig. 7 (a) When water is poured into the vessel, the contact line flows over the common paper with an origami frog; on the other hand, the contact line surrounds the superhydrophobic paper which then floats (b). White arrows indicate the contact line of water flow. (c) is the schematic for the supporting force analysis and (d) is the calculated plot of contact angle (θ) versus supporting force (Fsupporting) by eqn (1). | ||
A lotus-like support model is constructed by a large origami frog being placed on superhydrophobic paper (its area is 30 mm × 30 mm). They were together put into the bottom of an empty vessel. Water was poured into the vessel. Then we observe if the paper could float on the water. As expected, it cannot float any origami frogs (video S1†) and the common paper is immersed immediately when water flows through its surface (Fig. 7a). In contrast, it is interesting to notice that water does not pass through the superhydrophobic paper (Fig. 7b). After more water is added, the superhydrophobic paper really floats together with the origami frog (video S2†). However, the origami frog is so light that its weight cannot reflect the carrying capacity of the lotus-like paper. So its maximum floating force is measured as 48.7 ± 0.2 mN. This means that floating superhydrophobic paper can bear loading equivalent to ∼68 times its own weight (0.072 g, i.e. 0.71 mN). Force analysis is helpful to understand such great supporting force, Fsupporting, of the superhydrophobic paper (Fig. 7c):
| Fsupporting = Fs + Fb − Fg | (1) |
Fs = 4aγ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ
| (2) |
| Fb = a2ρgh | (3) |
| Fg = mg | (4) |
 | (5) |
We calculate the relationship between the supporting force and the CA of the paper in Fig. 7d. Note that there is a maximum supporting force when the CA value increases to a certain angle θc (here, i.e. ∼146°). It is deduced that the supporting force will be at a maximum one when the CA of the paper is beyond θc. Due to the high CA (161.2 ± 1.3°, beyond θc), our superhydrophobic paper can provide great support. Moreover, the calculated maximum supporting force (50.16 mN) is very close to our measurements.
Importantly, the flexible superhydrophobic paper has the advantage of long-term stably floating on water. It was examined by the non-standard tests of shaking the vessel with water. The paper together with an origami frog firstly floated on the vessel. We shook the vessel to see if the origami frog stably stood on the paper (video S3†). After shaking several times, the origami frog could still stably stand on the superhydrophobic paper, not immersed. We ascribe the good floating stability to its lotus-like flexibility. Fig. 8a shows the schematic comparisons between a rigid and flexible superhydrophobic substrate when they suffer a water wave. Clearly, one end of each substrate can be tilted along such a wave. For a rigid substrate, it is wholly tilted at the angle of α and then its other end develops a meniscus. The substrate would be immersed until its meniscus CA (θ) along the horizontal plane reach θm,c (i.e. θm,c = θe − α, where θe is equilibrium CA of the substrate). In contrast, the other end of the flexible substrate far from the wave is easy to bend. This bend decreases α to a lower value (β) and then the θm,c value of the meniscus rises, suggesting the flexible substrate becomes more difficult to sink. The flexible lotus-like paper therefore maintains better floating balance or stability. It is stressed that the edges of the superhydrophobic paper on water are tough (not bent) unless it suffers a dynamic impact or water wave, so the flexibility of the superhydrophobic paper does not affect the loading capacity when it is statically floating on water. Fig. 8b shows the duration effects on the maximum supporting capacity of the superhydrophobic papers. After floating on water surface for 30 days, the coated paper retains a supporting force of ∼47 mN, suggesting the good long-term supporting ability of the superhydrophobic paper.
Further, to offers insights into the practical use of the flexible superhydrophobic paper, we performed one simple wear test by pressing it firmly with a bare finger, as shown in Fig. 9a. After the coated paper was pressed, we deposited some 4 μl droplets to check their CAs. Clearly, a droplet still maintains a high CA (∼149°) on the pressed paper although it is less than the initial one (∼154°, Fig. 5b). The measurements for SA (32.1 ± 13.5°) display that the paper surface becomes stickier for a droplet after being pressed. Moreover, its water immersion absorption grows to ∼8 wt% for 10 min. The surface morphology of the pressed paper is also examined (Fig. S3†). Note that some agglomerations on the paper fibers are eliminated. So the wetting variations from the wear tests may result from contamination of the surface by grease/dust of finger and the partial damage of its microstructures. In any case, the slight decrease of CA, increase of SA and water absorption indicate that the superhydrophobicity of lotus-like paper is retained after wear tests. Furthermore, the pressed superhydrophobic paper together with the origami frog do not sink after floating for 30 days. These studies establish the good mechanical stability of the flexible superhydrophobic papers.
 | ||
| Fig. 9 (a) Optical images of some droplets deposited on the superhydrophobic paper and (b) variation of CA and SA before and after being pressed by one's first finger. | ||
4. Conclusions
The superhydrophobic papers with the PMMA-co-PS coating were successfully fabricated by solution-immersion method. Nanostructures from phase-separation of the polymer induce the high wetting adhesion of the coated paper. Inspired by the lotus leaf, the micro/nano micro-structures are constructed on the paper surface to reduce its adhesion by mixing the nano-sized silica particles into polymer solution. Superhydrophobic papers with low adhesion restrain the capillary absorption of water and maintain ultralow immersion absorption. Due to the low water absorption and high CA value, the superhydrophobic paper possesses a large supporting force. Moreover, the flexibility of superhydrophobic paper gives rise to its good dynamic floating stability. It is believed that the superhydrophobic papers can be applied as supporting materials in the field of fabricating aquatic micro-devices.Acknowledgements
This work was supported by the National Natural Science Foundation of China (51103033).Notes and references
- X. F. Gao and L. Jiang, Nature, 2004, 432, 36 CrossRef CAS PubMed.
- D. L. Hu, B. Chan and J. W. M. Bush, Nature, 2003, 424, 663 CrossRef CAS PubMed.
- D. L. Hu and J. W. M. Bush, Nature, 2005, 437, 733 CrossRef CAS PubMed.
- L. Jiang, X. Yao, H. Li, Y. Fu, L. Chen, Q. Meng and W. Hu, Adv. Mater., 2010, 22, 376 CrossRef CAS PubMed.
- F. Shi, J. Niu, J. Liu, F. Liu, Z. Wang, X. Q. Feng and X. Zhang, Adv. Mater., 2007, 19, 2257 CrossRef CAS.
- Q. Pan and M. Wang, ACS Appl. Mater. Interfaces, 2009, 1, 420 CAS.
- Q. Pan, J. Liu and Q. Zhu, ACS Appl. Mater. Interfaces, 2010, 2, 2026 CAS.
- F. Shi, Z. Q. Wang and X. Zhang, Adv. Mater., 2005, 17, 1005 CrossRef CAS.
- Y. S. Song, S. H. Suhr and M. Sitti, IEEE Int. Conf. Robot. Autom., 2006, 5, 2303 Search PubMed.
- X. Feng, X. Gao, Z. Wu, L. Jiang and Q. Zheng, Langmuir, 2007, 23, 4892 CrossRef CAS PubMed.
- B. Shin, H. Y. Kim and K. J. Cho, IEEE Int. Conf. Rob. Biomimetics, 2008, 127 Search PubMed.
- W. Barthlott and C. Neinhuis, Planta, 1997, 202, 1 CrossRef CAS.
- J. B. Boreyko and C. H. Chen, Phys. Rev. Lett., 2009, 103, 174502 CrossRef.
- L. Feng, S. Li, Y. Li, H. Li, L. Zhang, J. Zhai, Y. Song, B. Liu, L. Jiang and D. Zhu, Adv. Mater., 2002, 14, 1857 CrossRef CAS.
- A. Lafuma and D. Quéré, Nat. Mater., 2003, 2, 457 CrossRef CAS PubMed.
- B. Bhushan and Y. C. Jung, Prog. Mater. Sci., 2011, 56, 1 CrossRef CAS PubMed.
- K. Koch, B. Bhushan, Y. C. Jung and W. Barthlott, Soft Matter, 2009, 5, 1386 RSC.
- X. Zhang, F. Shi, J. Niu, Y. Jiang and Z. Wang, J. Mater. Chem., 2008, 18, 621 RSC.
- J. Zhang, J. Wang, Y. Zhao, L. Xu, X. Gao, Y. Zheng and L. Jiang, Soft Matter, 2008, 4, 2232 RSC.
- J. Liu, C. Bai, D. Jia, W. Liu, F. He, Q. Liu, J. Yao, X. Wang and Y. Wu, RSC Adv., 2014, 4, 18025 RSC.
- B. N. Sahoo and B. Kandasubramanian, RSC Adv., 2014, 4, 22053 RSC.
- Q. Xie, G. Fan, N. Zhao, X. Guo, J. Xu, J. Dong, L. Zhang and Y. Zhang, Adv. Mater., 2004, 16, 1830 CrossRef CAS.
- Z. Cheng, R. Hou, Y. Du, H. Lai, K. Fu, N. Zhang and K. Sun, ACS Appl. Mater. Interfaces, 2013, 5, 8753 CAS.
- G. Chitnis, Z. Ding, C. L. Chang, C. A. Savran and B. Ziaie, Lab Chip, 2011, 11, 1161 RSC.
- A. W. Martinez, S. T. Phillips, B. J. Wiley, M. Gupta and G. M. Whitesides, Lab Chip, 2008, 8, 2146 RSC.
- A. I. Neto, H. J. Meredith, C. L. Jenkins, J. J. Wilker and J. F. Mano, RSC Adv., 2013, 3, 9352 RSC.
- T. Wu, Y. Pan and L. Li, J. Colloid Interface Sci., 2010, 348, 265 CrossRef CAS PubMed.
- Z. Hu, X. Zen, J. Gong and Y. Deng, Colloids Surf., A, 2009, 351, 65 CrossRef CAS PubMed.
- S. Wang, M. Li and Q. Lu, ACS Appl. Mater. Interfaces, 2010, 2, 677 CAS.
- B. Kakade, R. Mehta, A. Durge, S. Kulkarni and V. Pillai, Nano Lett., 2008, 8, 2693 CrossRef CAS PubMed.
- D. Barona and A. Amirfazli, Lab Chip, 2011, 11, 936 RSC.
- H. Ogihara, J. Xie, J. Okagaki and T. Saji, Langmuir, 2012, 28, 4605 CrossRef CAS PubMed.
- B. Balu, V. Breedveld and D. W. Hess, Langmuir, 2008, 24, 4785 CrossRef CAS PubMed.
- S. Li, H. Xie, S. Zhang and X. Wang, Chem. Commun., 2007, 4857 RSC.
- A. G. Cunha and A. Gandini, Cellulose, 2010, 17, 875 CrossRef CAS.
- Y. L. Tai and Z. G. Yang, J. Mater. Chem., 2011, 21, 5938 RSC.
- X. Yu, T. Shi, G. Zhang and L. An, Polymer, 2006, 47, 1538 CrossRef CAS PubMed.
- Y. Ma, X. Cao, X. Feng, Y. Ma and H. Zou, Polymer, 2007, 48, 7455 CrossRef CAS PubMed.
- Z. Cheng, M. Du, H. Lai, N. Zhang and K. Sun, Nanoscale, 2013, 5, 2776 RSC.
- C. W. Extrand and S. I. Moon, Langmuir, 2009, 25, 992 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Graphs show the additional information concerning SEM image of common paper, schematic illustration for the different adhesion for a droplet, SEM image of superhydrophobic paper after the wear test and the videos showing that water flows on the common and superhydrophobic paper. See DOI: 10.1039/c4ra07842j |
| This journal is © The Royal Society of Chemistry 2014 |






