Preparation and use of combustion-derived Bi2O3 for the synthesis of heterocycles with anti-cancer properties by Suzuki-coupling reactions†
Sebastian Anushaa,
B. S. Anandakumara,
Chakrabhavi Dhananjaya Mohanb,
G. P. Nagabhushanaa,
B. S. Priyab,
Kanchugarakoppal S. Rangappab,
Basappa*a and
Chandrappa G. T*a
aDepartment of Chemistry, Central College Campus, Bangalore University, Bangalore-560 001, India. E-mail: salundibasappa@gmail.com; Tel: +91 8022961346
bDepartment of Studies in Chemistry, University of Mysore, Manasagangotri, Mysore-570 006, India. E-mail: gtchandrappa@yahoo.co.in; Tel: +91 8022961350
First published on 9th October 2014
Abstract
Bismuth oxide was synthesized via simple, rapid and energy efficient solution combustion synthesis (SCS) by employing sucrose as a fuel. This SCS-Bi2O3 was characterized by analytical techniques such as PXRD, SEM, EDX, UV-Visible and BET surface area measurement. Using the prepared SCS-Bi2O3, several classes of heterocyclic compounds were synthesized in good yields via Suzuki-coupling reaction in aqueous medium. Interestingly, the recovered SCS-Bi2O3 could be reused three times without a significant loss of catalytic activity. Further, the synthesized compounds were tested for cytotoxic activity against a human hepatoma cancer cell line (HepG2). Among these, compound 3n effectively inhibited the proliferation of these cells with an IC50 value of 8.4 μM. Thus, this paper describes the preparation of highly effective Bi2O3, which can be used for synthesizing various classes of heterocycles, including those having anti-cancer property.
Introduction
In the past decade a number of methods such as solid-state reactions,1 sonochemical routes,2 thermolysis,3 hydrothermal methods4 and solution combustion synthesis (SCS)5,6 have been developed for the synthesis of Bi2O3. SCS is the simpler route to develop metal oxides compared to other methods.7,8 The SCS method has several advantages including rapid synthesis at ambient atmosphere, low cost with a great potential scale up and is eco-friendly i.e. complete conversion to non-toxic gases. SCS is also useful for producing homogeneous, porous and crystalline fine powders with a large surface area.9Metal oxides are extensively used in many organic transformations especially in the heteroaryl preparation via cross coupling reactions. Methods reported for the synthesis of heteroaryls have one or more advantages as well as disadvantages such as involvement of expensive reagents, formation of byproducts, thermal instability, use of toxic solvents, requirement of excess of reagents/catalysts, laborious work up procedure and use of extensive catalyst. To overcome these drawbacks, the development of an alternate, milder, and cleaner procedure is highly desirable.10 It should be efficient, and involve an easy work-up procedure which affords greater yields of desired products in shorter reaction time.11,12
On the other hand, heteroaryls has been recognized as a privileged structure by medicinal chemists as they are molecular scaffolds with versatile binding properties, which exhibit good drug-like properties.13–16 The biaryl motif has shown activity across a wide range of therapeutic classes which include antifungal, anti-inflammatory, anti-rheumatic, antitumor and antihypertensive agents.17,18
Herein, we report a combustion derived Bi2O3 by employing cost effective sucrose as fuel and to the best of our knowledge, this is the first report on synthesis of Bi2O3 using sucrose as fuel via SCS method. However, there are reports on metal oxides other than Bi2O3 prepared using sucrose19,20 as fuel via SCS. In this paper, we emphasize not only on the basic characteristics of Bi2O3 but also on high surface area which makes it a promising reagent in organic synthesis. The large surface area can provide a greater number of active sites than the low coordinated oxide sites (edges, corners) and lattice defects (cation and anion vacancies).21,22 Also we report the use of SCS-Bi2O3 in combination with [1,1′-bis(diphenylphosphino)ferrocene]dichloropalladium(II) (Pd(dppf)Cl2) as an effective base–catalyst system for coupling substituted pyridine halides with boronic acids. These reactions were carried out in water under aerobic conditions using tetra-n-butylammonium bromide (TBAB) as an additive.23–25 This system is of due importance as it avoids the use of any organic solvents and easy separation of product from the reusable catalyst which greatly facilitates the work up.26
Result and discussion
Characterisation of SCS-Bi2O3
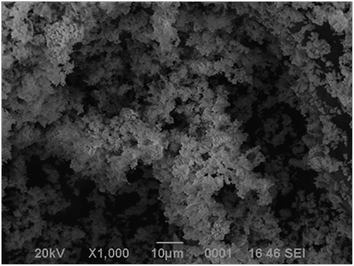
Fig. 1 shows powder X-ray diffraction (PXRD) pattern of prepared SCS-Bi2O3. All the characteristic peaks in the pattern can be indexed to a monoclinic phase of α-Bi2O3 with lattice parameters a = 5.8480, b = 8.1660, c = 7.5100 and β = 113 (JCPDF card no. 00-027-0053). The crystallite size calculated using Scherer's formula is found to be 65–70 nm.The scanning electron microscope (SEM) micrograph of Bi2O3 powders (Fig. 2) shows that the powder is porous and agglomerated. The pores and voids can be attributed to the large amount of gas escaping during the combustion. The energy dispersive X-ray spectroscopy (EDX) spectrum of Bi2O3 synthesised shown in (ESI Fig. 1†) exhibit peaks for Bi and O elements confirming the presence of Bi2O3. Brunauer–Emmett–Teller (BET) nitrogen adsorption–desorption isotherm of SCS-Bi2O3 is shown in (ESI Fig. 2†). The surface area of SCS-Bi2O3 was calculated by the BET method. SCS-Bi2O3 has a large surface area, f 24 m2 g−1, compared to commercial Bi2O3, (8 m2 g−1). During combustion, the larger surface area attributed to the liberation of gaseous products such as water, carbon dioxide and gaseous nitrogen. The band gap energy of SCS-Bi2O3 was calculated (2.88 eV) by using UV-Visible spectra with Mott–Schottky plot as shown in Fig. 3 (the absorbance spectra is shown in ESI Fig. 3†).
Optimisation of reaction conditions
The reaction was studied on 2-amino-3-bromopyridine using 4-trifluromethylphenylboronic acid (Scheme 1). Efforts were focussed on screening different palladium catalysts in various solvents and the results are summarised in (Table 1). It was found that the ideal system for the cross coupling reaction is combustion derived Bi2O3 (SCS-Bi2O3)/Pd(dppf)Cl2 in water. The reactions with other palladium catalysts were not proceeding in water and low conversions were observed in 80% dioxane. The most robust reaction was achieved by the use of 0.8 equivalence of the SCS-Bi2O3 and 0.03 equivalence of the Pd(dppf)Cl2 in aqueous medium. These results indicate that the SCS-Bi2O3 is effective in aqueous medium compared to organic–aqueous medium.| Catalyst | Solvent | Yield (%) |
|---|---|---|
a Conditions: 2-amino-3-bromopyridine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4-trifluromethylphenylboronic acid 4-trifluromethylphenylboronic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SCS-Bi2O3 SCS-Bi2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pd catalyst Pd catalyst![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TBAB = 1 TBAB = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.05 1.05![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8 0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.03 0.03![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 (mmol), solvent = 10 mL per mmol substrate, 110 degrees, 8 h.b Isolated yield after column chromatography; SCS-combustion derived, NR – no reaction. 0.5 (mmol), solvent = 10 mL per mmol substrate, 110 degrees, 8 h.b Isolated yield after column chromatography; SCS-combustion derived, NR – no reaction. |
||
| Pd(dppf)Cl2 | 80% dioxane | 68b |
| Pd(dppf)Cl2 | Water | 88 |
| Pd(PPh3)4 | 80% dioxane | 55b |
| Pd(PPh3)4 | Water | NR |
| Pd2dba3 | 80% dioxane | NR |
| Pd2dba3 | Water | NR |
| Pd(PPh3)2Cl2 | 80% dioxane | 61b |
| Pd(PPh3)2Cl2 | Water | NR |
| Pd(OAc)2 | 80% dioxane | NR |
| Pd(OAc)2 | Water | NR |
Influence of SCS-Bi2O3 on Suzuki coupling
SCS-Bi2O3 acts as an effective base in the reaction of various 2- or 3-halopyridines with a wide range of aryl as well as heteroaryl boronic acids. The reaction was investigated on both bromides and iodides under the optimized condition (Scheme 2) and the results were summarised in Table 2.| Entry | Halopyridine (1a–1h) | Boronic acid (2a–2j) | Product (3a–3r) | Time (h) | Yield (%) | IC50 (μM) |
|---|---|---|---|---|---|---|
a Conditions: substrate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) boronic acid boronic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SCS-Bi2O3 SCS-Bi2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pd(dppf)Cl2 Pd(dppf)Cl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TBAB = 1 TBAB = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.05 1.05![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8 0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.03 0.03![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 (mmol), H2O = 10 mL per mmol substrate, 110 degrees.b Isolated yield after column chromatography. 0.5 (mmol), H2O = 10 mL per mmol substrate, 110 degrees.b Isolated yield after column chromatography. |
||||||
| 3a |  |
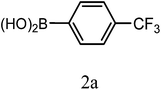 |
 |
5 | 88 | >50 |
| 3b |  |
 |
 |
5 | 80 | >50 |
| 3c |  |
 |
 |
4 | 86 | 16.6 ± 1.9 |
| 3d |  |
 |
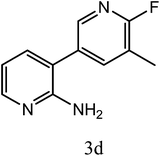 |
6 | 68b | >50 |
 |
 |
 |
4 | 69b | >50 | |
| 3e | 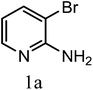 |
 |
 |
8 | 70b | >50 |
 |
 |
 |
4 | 72b | >50 | |
| 3f |  |
 |
 |
4 | 89 | 44.9 ± 3.6 |
| 3g |  |
 |
 |
4 | 87 | >50 |
| 3h |  |
 |
 |
3 | 78 | >50 |
| 3i |  |
 |
 |
4 | 83 | >50 |
| 3j | 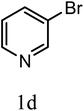 |
 |
 |
4 | 81 | >50 |
| 3k |  |
 |
 |
4 | 79 | >50 |
| 3l |  |
 |
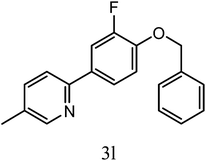 |
5 | 84 | 35.3 ± 2.3 |
| 3m |  |
 |
 |
8 | 87 | 47.2 ± 2.1 |
| 3n | 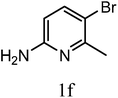 |
 |
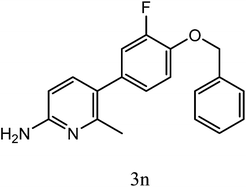 |
9 | 81 | 8.4 ± 2.8 |
| 3o |  |
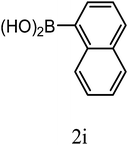 |
 |
9 | 76 | 20.8 ± 2.5 |
| 3p | 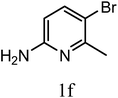 |
 |
 |
9 | 66b | >50 |
| 3q |  |
 |
 |
No reaction | ||
| 3r | 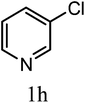 |
 |
 |
No reaction | ||
Majority of reactions completed within 10 h with product isolation by simple filtration of the undissolved base–catalyst from the reaction media. In many cases pure product was afforded in excellent yields by crystallisation of the crude product using ethyl acetate and hexane mixture. All the reactions on 2-amino-3-bromopyridine were fast and prolongation of reaction time was observed in the case of 2-amino-3-bromo-6-methylpyridine. Reactions of iodides were faster than corresponding bromides but the yields were comparable in most of the cases. With regard to chemo selectivity this base–catalyst system is not compatible with chloro substituted pyridines and is attributed to its reluctance to participate in oxidative addition as high energy is required for this first step of the catalytic cycle.27 We also observed a loss of yield in the case of ortho substituted and heteroaryl boronic acids28 with no improvement in the reaction conversion on prolonging the reaction time up to 14 h. (Table 2, entries 3d, 3e, 3p). Thus SCS-Bi2O3 is tolerant of electronic variations in halopyridines as well as boronic acid components with cleaner reactions, ease of isolation and better yields.
Re-usability of the base–catalyst system
Experiments were performed to study the recyclability of the base–catalyst system employing 2-amino-3-bromopyridine with 4-trifluromethylphenylboronic acid to yield the compound 3a (Scheme 1). We observed the significant reduction in the yield of the product after third run (Table 3). After the completion of the reaction, aqueous layer containing the product was separated and the base–catalyst remaining was reused for another run by adding fresh 2-amino-3-bromopyridine, 4-trifluromethylphenylboronic acid and TBAB. It is important to stress that this system was readily recyclable for three times and the isolated yields were above 70%.Biology
Newly synthesized compounds were evaluated in vitro for their anticancer property by MTT assay as described earlier.15 It is a photometric assay that measures the reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide by enzyme mitochondrial succinate dehydrogenase. Most compounds displayed different degree of cytotoxicity. Half maximal inhibitory concentration (IC50) of the compounds 3a to 3p was summarized in Table 2. Among the newly synthesized compounds, 3c, 3o and 3n showed significant cytotoxic effect on HepG2 cells with the IC50 of 16.6 μM, 20.8 μM and 8.4 μM respectively, whose activity is comparable to the known anticancer drug Sorafenib. 3f, 3m and 3l are moderately active with the IC50 of 44.9 μM, 47.2 μM and 35.3 μM respectively. Other members are less active against HepG2 cells with IC50 higher than 50 μM.Experimental section
Materials and method
Bismuth(III) nitrate pentahydrate of the purity of 99%, and commercial Bi2O3 powder of the purity of 99% were purchased from Merck Chemicals, India. Commercially available sucrose was used and all organic chemicals used were purchased from Sigma-Aldrich. Distilled water was collected from Merck Millipore Direct-Q 3, 5, 8 Ultrapure Water Systems. MTT and SDS were purchased from Sigma-Aldrich (St. Louis, MO). Dulbecco's Modified Eagle Medium (DMEM), fetal bovine serum (FBS), antibiotic–antimycotic mixture were obtained from Invitrogen. Powder X-ray diffraction patterns were obtained by a Philips X'pert PRO X-ray diffractometer using CuKα radiation (λ = 1.54056 Å). Morphologies of combustion derived powders were examined using a JEOL-JSM-6490 LV scanning electron microscope (SEM), Nitrogen adsorption–desorption measurements were carried out at 77 K using a gas sorption analyzer Quanta chrome corporation NOVA 1000. The surface area was calculated using the BET method. UV-Vis measurements were performed using a UV-Vis spectrophotometer (Shimadzu UV-3101). All IR spectra were obtained in KBr disc on a Shimadzu FT-IR 157 Spectrometer. 1H and 13C NMR spectra were recorded on a Bruker WH-200 (400MZ) spectrometer in CDCl3 or DMSO-d6 as solvent, using TMS as an internal standard and chemical shifts are expressed as ppm. Mass spectra were determined on a Agilent LC-MS and the elemental analyses were carried out using an Elemental Vario Cube CHNS rapid Analyzer. The progress of the reaction was monitored by TLC pre-coated silica gel G plates.Cell lines
HepG2 was a kind gift of Dr Gautam Sethi at the National University of Singapore. HepG2 cells were cultured in Dulbecco's Modified Eagle Medium (DMEM) containing 1× antibiotic–antimycotic solution with 10% FBS.Synthesis of SCS-Bi2O3
In a petridish, solution of Bi(NO3)3·5H2O was prepared by dissolving 5 g Bi(NO3)3·5H2O in 4 mL of 6 M HNO3 and the excess acid was removed by heating on a hot plate to this sucrose solution was added by dissolving 1.106 g of sugar in 25 mL of distilled water. An aqueous solution containing the above mixture of Bi(NO3)3·5H2O as an oxidizer (O) and sugar (F) (corresponding F/O ratio Ø = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)29,30 was taken in a petridish. Excess water was allowed to evaporate by heating the solution on a hot plate until the formation of a yellow viscous gel. Then the petridish was introduced into a muffle furnace maintained at 400 ± 10 °C. Initially, the viscous gel underwent dehydration and commenced smoldering combustion, which appeared at one end and propagated through the mass within one minute. A voluminous and porous nanocrystalline product was obtained.
1)29,30 was taken in a petridish. Excess water was allowed to evaporate by heating the solution on a hot plate until the formation of a yellow viscous gel. Then the petridish was introduced into a muffle furnace maintained at 400 ± 10 °C. Initially, the viscous gel underwent dehydration and commenced smoldering combustion, which appeared at one end and propagated through the mass within one minute. A voluminous and porous nanocrystalline product was obtained.
Typical procedure for the synthesis of novel heterocyclic compounds
To a solution of halopyridine (1a–1h) (0.3 mmol) in 10 mL of distilled water in a sealed tube was added boronic acid (2a–2j) (0.32 mmol), SCS-Bi2O3 (0.24 mmol), TBAB (0.15 mmol) and [1,1′-bis(diphenylphosphino)ferrocene]dichloropalladium(II) (0.009 mmol). The reaction mixture was heated to 110 °C for the required hours. The reaction was then cooled to room temperature and filtered to remove the base and catalyst. The product was extracted from the water layer by 3 × 5 mL ethyl acetate, dried with magnesium sulfate, filtered, and concentrated in vacuum. The crude product was purified by recrystallisation using ethyl acetate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane. All new compounds exhibited spectral properties consistent with the assigned structures and were fully characterized by their spectroscopic data (1H, IR, Mass, elemental and 13C NMR analysis) (ESI-4 to ESI-19†).
hexane. All new compounds exhibited spectral properties consistent with the assigned structures and were fully characterized by their spectroscopic data (1H, IR, Mass, elemental and 13C NMR analysis) (ESI-4 to ESI-19†).
MTT assay
The anti-proliferative activity of compounds (3a to 3p) against HCC cells was determined by the MTT dye uptake method as described previously.31 Briefly, the cells (2.5 × 104 per mL) were incubated in triplicate in a 96-well plate in the presence or absence of different concentrations of compounds in a final volume of 0.2 mL for indicated time intervals at 37 °C. Thereafter, 20 μL MTT solution (5 mg mL−1 in PBS) was added to each well. After a 2 h incubation at 37 °C, 0.1 mL lysis buffer (20% SDS, 50% dimethyl-formamide) was added; incubation was continued overnight at 37 °C; and then the optical density (OD) at 570 nm was measured by Tecan plate reader.Conclusion
Combustion derived Bi2O3 prepared and used for the synthesis of heteroaryls using Suzuki coupling reactions in aqueous medium. The base–catalyst system can also be recovered and reused up to three times with no significant loss of catalytic activity. Furthermore, the newly synthesized compounds were tested for its cytotoxic effects against human hepatoma cancer cells (HepG2) which revealed that the described application is effective in developing reagents for the chemical biology programme.Acknowledgements
This research was supported by University Grants Commission (41-257-2012-SR), Vision Group Science and Technology, Department of Science and Technology (no. SR/FT/LS-142/2012) to Basappa. AS, CDM and B thank, UGC, DST-INSPIRE and Pavate fellowships respectively. The authors are thankful to prof. Y. S. Bhat, Bangalore Institute of Technology for surface area measurement.References
- R. Roth and J. Waring, Am. Mineral., 1963, 48, 1348–1356 CAS.
- L. Zhang, W. Wang, J. Yang, Z. Chen, W. Zhang, L. Zhou and S. Liu, Appl. Catal., A, 2006, 308, 105–110 CrossRef CAS PubMed.
- L. Zhou, W. Wang, H. Xu, S. Sun and M. Shang, Chem.–Eur. J., 2009, 15, 1776–1782 CrossRef CAS PubMed.
- Q. Yang, Y. Li, Q. Yin, P. Wang and Y.-B. Cheng, Mater. Lett., 2002, 55, 46–49 CrossRef CAS.
- R. Jha, R. Pasricha and V. Ravi, Ceram. Int., 2005, 31, 495–497 CrossRef CAS PubMed.
- M. Anilkumar, R. Pasricha and V. Ravi, Ceram. Int., 2005, 31, 889–891 CrossRef CAS PubMed.
- A. K. Alves, C. P. Bergmann and F. A. Berutti, Novel Synthesis and Characterization of Nanostructured Materials, Springer, 2013 Search PubMed.
- S. T. Aruna and A. S. Mukasyan, Curr. Opin. Solid State Mater. Sci., 2008, 12, 44–50 CrossRef CAS PubMed.
- K. C. Patil, S. Aruna and T. Mimani, Curr. Opin. Solid State Mater. Sci., 2002, 6, 507–512 CrossRef CAS.
- Z. Zhang, J. Mao, R. Wang, F. Wu, H. Chen and B. Wan, J. Mol. Catal. A: Chem., 2006, 243, 239–243 CrossRef CAS PubMed.
- K. C. Basappa and K. Rangappa, Bioorg. Med. Chem. Lett., 2004, 14, 3279–3281 CrossRef PubMed.
- K. Mantelingu, M. Sadashiva and K. Rangappa, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2004, 43, 1954–1957 Search PubMed.
- Basappa, S. Murugan, C. V. Kavitha, A. Purushothaman, K. G. Nevin, K. Sugahara and K. S. Rangappa, Cancer Lett., 2010, 297, 231–243 CrossRef CAS PubMed.
- A. Y. Sukhorukov, A. C. Nirvanappa, J. Swamy, S. L. Ioffe, S. Nanjunda Swamy, Basappa and K. S. Rangappa, Bioorg. Med. Chem. Lett., 2014, 24, 3618–3621 CrossRef CAS PubMed.
- H. K. Keerthy, C. D. Mohan, K. S. Siveen, J. E. Fuchs, S. Rangappa, M. S. Sundaram, F. Li, K. S. Girish, G. Sethi, B. Basappa, A. Bender and K. S. Rangappa, J. Biol. Chem., 2014 DOI:10.1074/jbc.m114.593855.
- C. M. Shivaprasad, S. Jagadish, T. R. Swaroop, C. D. Mohan, R. Roopashree, K. S. S. Kumar and K. S. Rangappa, Eur. J. Chem., 2014, 5, 91–95 CrossRef.
- K. Sugahara, K. N. Thimmaiah, H. K. Bid, P. J. Houghton and K. S. Rangappa, PLoS One, 2012, 7, e39444 CrossRef.
- B. S. Priya, C. Anil Kumar, S. Nanjunda Swamy, Basappa, S. Naveen, J. Shashidhara Prasad and K. S. Rangappa, Bioorg. Med. Chem. Lett., 2007, 17, 2775–2780 CrossRef CAS PubMed.
- R. Ianoş, A. Tăculescu, C. Păcurariu and I. Lazău, J. Am. Ceram. Soc., 2012, 95, 2236–2240 CrossRef PubMed.
- S. K. Ghosh, A. Prakash, S. Datta, S. K. Roy and D. Basu, Bull. Mater. Sci., 2010, 33, 7–16 CrossRef CAS PubMed.
- C. Sousa, G. Pacchioni and F. Illas, Surf. Sci., 1999, 429, 217–228 CrossRef CAS.
- M. Sterrer, T. Berger, O. Diwald and E. Knozinger, J. Am. Chem. Soc., 2003, 125, 195–199 CrossRef CAS PubMed.
- J. Zhou, X. Guo, C. Tu, X. Li and H. Sun, J. Organomet. Chem., 2009, 694, 697–702 CrossRef CAS PubMed.
- S. S. Soomro, C. Röhlich and K. Köhler, Adv. Synth. Catal., 2011, 353, 767–775 CrossRef CAS.
- Y. M. Yamada, K. Takeda, H. Takahashi and S. Ikegami, J. Org. Chem., 2003, 68, 7733–7741 CrossRef CAS PubMed.
- C. Dupuis, K. Adiey, L. Charruault, V. Michelet, M. Savignac and J.-P. Genêt, Tetrahedron Lett., 2001, 42, 6523–6526 CrossRef CAS.
- W. Shen, Tetrahedron Lett., 1997, 38, 5575–5578 CrossRef CAS.
- P.-P. Zhang, X.-X. Zhang, H.-X. Sun, R.-H. Liu, B. Wang and Y.-H. Lin, Tetrahedron Lett., 2009, 50, 4455–4458 CrossRef CAS PubMed.
- B. Nagappa and G. T. Chandrappa, Microporous Mesoporous Mater., 2007, 106, 212–218 CrossRef CAS PubMed.
- G. P. Nagabhushana, G. Nagaraju and G. T. Chandrappa, J. Mater. Chem. A, 2013, 1, 388–394 CAS.
- B. Kumar, S. Paricharak, K. R. Dinesh, K. Sivaraman Siveen, J. Fuchs, S. Rangappa, C. D. Mohan, N. Mohandas, A. P. Kumar, G. Sethi, A. Bender, S. Basappa and K. S. Rangappa, RSC Adv., 2014 10.1039/c4ra08713e.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra07839j |
| This journal is © The Royal Society of Chemistry 2014 |