CO2 diffusion in n-hexadecane investigated using magnetic resonance imaging and pressure decay measurements
Abstract
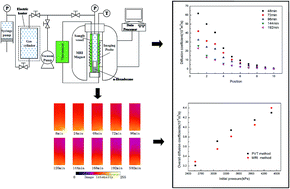
A method combining magnetic resonance imaging (MRI) and dual-chamber pressure decay (i.e., the pressure–volume–temperature method) was used to measure the diffusivity of CO2 in bulk n-hexadecane, a representative oil, at 21 °C. Images of the proton density of n-hexadecane were obtained by MRI using a high magnetic field and high resolution, and the pressure decay was recorded. Overall diffusion coefficients of CO2 were calculated from both pressure decay and MRI intensity data. The two results showed good agreement. MRI was successfully used to study the diffusivity of CO2 in n-hexadecane. The change of image density (i.e., proton density) demonstrates that the oil density decreases as CO2 diffuses into it. The finite volume method was applied to the images obtained by MRI, allowing the diffusion coefficient of CO2 to be directly obtained. MRI methods can measure the unsteady-state local diffusion coefficient and overall diffusion coefficient of such systems.

 Please wait while we load your content...
Please wait while we load your content...