A novel probe for selective colorimetric sensing of Fe(ii) and Fe(iii) and specific fluorometric sensing of Fe(iii): DFT calculation and logic gate application†
Abstract
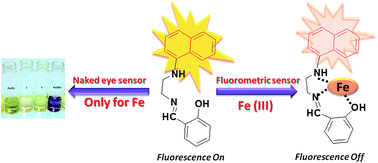
A novel fluorescent probe 1 (2-((2-(naphthalen-1-ylamino)ethylimino)methyl)phenol) has been synthesized and characterized by various spectroscopic methods. Probe 1 was found to be highly selective for iron over tested metal ions. The naked eye detection of iron is useful for the discrimination of the +2 and +3 oxidation state while fluorescence studies conclude selective and specific sensitivity towards Fe(III).

 Please wait while we load your content...
Please wait while we load your content...