Comparative study of the bio-remediation of eutrophic river water, using two biofilm processes
Haijing Yua and
Wenping Cao*b
aSchool of Municipal and Environmental Engineering, Henan University of Urban Construction, Henan 467036, P. R. China. E-mail: wenpingcao2013@163.com
bSchool of Environmental Engineering, Xuzhou Institute of Technology, Jiangsu 221000, P. R. China
First published on 24th September 2014
Abstract
Filamentous bamboo and plastic filling were used as biofilm carriers for the bio-remediation of nitrogenous compounds from eutrophic river water. Two corresponding biofilm reactors were developed: a filamentous bamboo reactor (FBR) and a plastic filling reactor (PFR). Experimental results indicated that the average removal rates of total nitrogen (TN), ammonium nitrogen (NH4+-N), nitrate nitrogen (NO3−-N), nitrite nitrogen (NO2−-N), chemical oxygen demand using KMnO4 as oxidizer (CODMn) and chlorophyll A were 63.86%, 47.80%, 64.75%, 20.00%, 63.50% and 58.36% for FBR, and 11.29%, 18.24%, 43.90%, −165%, 9.56% and 15.25% for PFR, respectively. Statistically significant differences between FBR and PFR (p < 0.05) were noted in TN, NH4+-N, NO3−-N, NO2−-N and CODMn. The results showed that NO2−-N was associated with accumulation phenomena in the PFR. It was also noted that the observed diversity of microorganisms (Protozoa and Metazoa) and the biomass of nitrifying bacteria and denitrifying bacteria were higher on the filamentous bamboo than that on the plastic filling (p < 0.05). These results suggest that filamentous bamboo may be a potential carbon source that could be used for glucose-replacement during de-nitrification.
Introduction
The eutrophication of inland water is a result of human activities such as rapid urbanization, industrialization and intensive agricultural production.1 Eutrophication in surface water bodies, such as lakes and reservoirs, can lead to a reduction in the biological diversity and recreational value of natural water bodies and water purification capacity, which can have a negative impact on human health. Bio-remediation, including in situ and ex situ remediation, can be used as an effective means of purifying eutrophic surface waters. Many in situ remediation processes — such as those associated with ecological floating bed techniques and constructed wetlands — have been developed for the purpose of bio-remediation of eutrophic surface waters. Satisfactory remediation results have been obtained, which are often associated with the manufacture of plant products that can be used as animal and human food or be processed into bio-gas, bio-fertilizers and bio-materials.2 Floating bed techniques also have the unique advantage of occupying no land area. Unfortunately, these processes are prone to unpredictable failures due to low temperature, limited phyto-uptake and restricted standing biomass, all of which are affected by low water transparency.3A number of bio-film reactor techniques for remediation of eutrophic river water have recently been developed and these have contributed to the remediation of eutrophic river water. These techniques have a number of advantages: land and energy saving, greater biomass concentration, flexible operation, lower sensitivity to toxicity, and greater volumetric loading.4 The type of carrier used for biofilm growth directly influences treatment efficiency and energy consumption.5,6 Research into this field has previously focused on the use of inert bio-carriers, including plastic material and light ceramsite, for the bio-remediation of eutrophic water bodies. Only a few studies have focused on the use of biodegradable materials as bio-carriers.
Certain solid carbon sources can function as a replenishment carbon substrate base for bio-denitrification as well as a biofilm carrier in a process that has been referred to as “solid phase de-nitrification (SPD)”. Various solids have been evaluated as useful solid carbon sources for this purpose: newspapers, unprocessed cotton fiber,7 the bark of various trees,8 hornbeam wood, pine shavings, sugar and sugar cane, water-insoluble biodegradable polymers, and synthetic polyester granules.9 Previous studies have indicated certain disadvantages associated with some of these carbon sources, due to high costs,8,10,11 their toxicity to microorganisms,10 or — in the case of wheat straw — because of poor mechanical strength.12
Filamentous bamboo does not have any of the above-mentioned disadvantages and contains many organic substances that could potentially be used as electron donors by denitrifying bacteria.13
The initial objectives of our study were to assess the bioremediation efficiency of the filamentous bamboo reactor (FBR) and the plastic filling reactor (PFR) in terms of chemical oxygen demand using KMnO4 as oxidizer (CODMn) and chlorophyl a (Chl-a) and a reduction in the levels of total nitrogen (TN), ammonium nitrogen (NH4+-N), nitrate nitrogen (NO3−-N) and nitrite nitrogen (NO2−-N), and compare the differences between the inert bio-carrier (plastic filling) and the biodegradable bio-carrier (filamentous bamboo) in terms of removing the nitrogenous compounds when bio-films was used as a bioremediation method for the treatment of eutrophic river water. The second objective was to examine the potential use of filamentous bamboo as a carbon source, during the process of de-nitrification.
Materials and methods
Bio-carriers
The values of porosity, specific surface area of filamentous bamboo and plastic filling were measured by the surface analyzer (V-Sorb 2800, China), the bulk density was self-measured by the ratio of the bulk quantities (kg) and the bulk volume (L).
Procedures
Experiments were carried out in two parallel sequencing batch bio-film reactors (SBBRs) each with 9 cm inner diameter, a height of 45 cm, and a working volume of 2.4 L. Both reactors were made of polymethyl methacrylate. Filamentous bamboo and plastic filling were chosen as bio-carriers for the reactors, with a filling ratio of about 30%. The specific surface area in each reactor was thus similar although the bio-carrier materials were different. The two reactors were developed and operated in batch mode under similar conditions. The experimental study was carried out at a water temperature of 19.0 ± 1.5 °C and a dissolved oxygen (DO) concentration of ≥3.5 mg L−1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 5
P = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, while glucose, or bamboo, was used as a carbon source during de-nitrification. The experiment was carried out in batches at 35 °C and 120 rpm.
1, while glucose, or bamboo, was used as a carbon source during de-nitrification. The experiment was carried out in batches at 35 °C and 120 rpm.The NO3−-N removal rate was compared under three de-nitrification systems, described below.
(1) Flask A: 121.5 mg NaNO3 and 15.5 mg KH2PO3 were dissolved in 150 mL tap water and 100 mL domesticated sludge.
(2) Flask B: 121.5 mg NaNO3, 15.5 mg KH2PO3 and 50 mg glucose were dissolved in 150 mL tap water and 100 mL domesticated sludge.
(3) Flask C: 121.5 mg NaNO3 and 15.5 mg KH2PO3 were dissolved in 150 mL tap water and 10 g filamentous bamboo, with steady-state biofilms.
Analytical methods
Water samples were collected at regular intervals and tested within 2 h of collection. All water samples were filtered through a 0.45 μm membrane. All compositional analyses in the study were performed in triplicate and the data are expressed as the mean ± the standard deviation. NH4+-N, NO2−-N, NO3−-N and Chl-a content were determined with an ion chromatograph analyzer (model: PIC-10A, Instrument Co., Ltd. Puren, Qingdao, China); an ultraviolet-visible spectrophotometer (Shimadzu UV2450, Japan) was used to measure TN, and CODMn was analyzed according to standard methods.14 Microscopic examination was carried out by optical microscope (model: XSD-36XC). The concentrations of bacteria, nitrifying bacteria, denitrifying bacteria and biomass weight, were analyzed according to the methods of Cao et al.15 The biofilm thickness was measured according to the methods of Tanyolac and Beyenal.16Statistical analyses
Treatment methods were compared using one-way analysis of variance (ANOVA) and the least significant difference (LSD) procedure was used for the purpose of mean comparisons, using a significance level of p = 0.05. Statistical analyses were performed with SPSS Base 19.0 statistical software (SPSS Inc., Chicago, IL, USA).Results
Biomass comparison between methods using filamentous bamboo and plastic filling as substrates
Many species of microorganism were observed on both the filamentous bamboo and the plastic filling. Microscopic examination indicated that species variability, in terms of Protozoa and Metazoa, observed on the filamentous bamboo was significantly higher than that on the plastic filling, and the biomass on the filamentous bamboo was also higher than that on the plastic filling. The average density of the attached nitrifying bacteria and denitrifying bacteria on filamentous bamboo was 8.1 × 108 cfu mL−1 and 9.2 × 107 cfu mL−1, respectively, while that on plastic filling was 3.6 × 108 cfu mL−1, and 2.3 × 107 cfu mL−1, respectively. The biomass of microorganisms on the FBR was statistically significant different to that on the PFR (p < 0.05) and the quantities of biofilm on filamentous bamboo and plastic filling were respectively 2.02 g m−2 and 0.98 g m−2. The reaction rate within the biofilm was also found to increase as the biofilm density increased.17,18Mean biofilm thickness on different bio-carriers
Mean biofilm thickness on the filamentous bamboo and plastic fillings were observed. Measurements of biofilm depth revealed that, following 3 days of incubation, the biofilm depth was similar on the two surfaces examined, with an average thickness of 15–18 μm. Following 7 days of incubation, the biofilm thickness on the plastic filling surfaces increased by approximately two fold, with an average thickness of 28–33 μm, while the average thickness of biofilm on the filamentous bamboo surface was 62–81 μm. It was noted that the biofilm which formed on the filamentous bamboo after 7 days was more than four times thicker than the biofilm formed after 3 days. Compared with the biofilm thickness results after 7 days, after 28 days of incubation the biofilm thickness on the plastic filling surfaces had increased by approximately 1.5 fold, with an average thickness of 43–51 μm, but the biofilm on the filamentous bamboo surface had an 11-fold increase, with an average thickness of 365–494 μm. It was also noted that higher surface roughness and biodegradable performance induced a thicker biofilm.19 Meanwhile, it was noted that the level of DO that could be transported into biofilm via diffusion is an important limiting factor in this process. This is affected by Fick's law, due to the decreased effective diffusion coefficient which are helpful for more complex biofilm system and more abundant microbial species,16,20,21 resulting in a longer microorganisms chain on filamentous bamboo.22,23Effects of two materials on CODMn removal efficiency
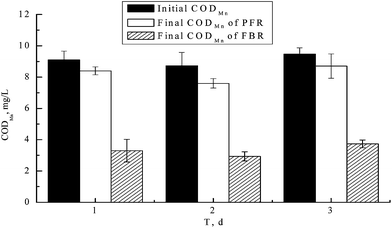
Results obtained from the FBR and PFR, when operated under similar conditions, are outlined in Fig. 1.A comparison of results indicates that the final concentrations of CODMn in the FBR were lower, and less variable, than those obtained in the PFR. When the initial mean CODMn was 9.10 ± 0.60 mg L−1, the corresponding final mean CODMn of FBR and PFR were 3.32 ± 0.42 mg L−1 and 8.23 ± 0.45 mg L−1, respectively. The average removal rate of CODMn was 63.5% and 9.56%, respectively. Relative to the PFR, the mean CODMn removal rate of the FBR increased by 53.94%. There were statistically significant differences between results obtained from the FBR and the PFR (p < 0.05).
Unlike the situation noted when using plastic filling (inert bio-carrier), the filamentous bamboo (natural bio-carrier) could be decomposed during water purification, resulting in a thicker biofilm on the filamentous bamboo. The thicker biofilm facilitates anaerobic conditions, so the filamentous bamboo is beneficial in terms of forming a richer microbial community and a higher rate of organic matter biodegradation.15
Effects of two materials on nitrogenous compounds removal efficiency
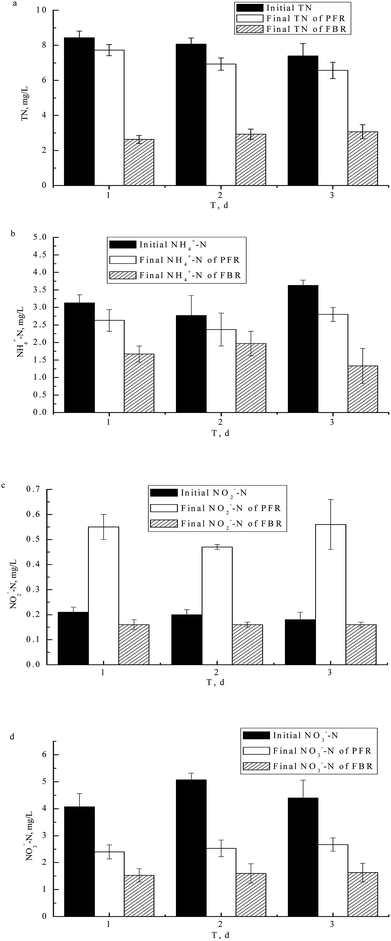
As can be seen in Fig. 2(a) and (b), the influent TN and NH4+-N concentrations were respectively 7.40–8.43 mg L−1 and 2.77–3.63 mg L−1, the effluent concentrations of TN in the FBR and PFR were respectively 2.63–3.07 mg L−1 and 6.57–7.73 mg L−1, and the effluent concentrations of NH4+-N in the FBR and PFR were respectively 1.33–1.97 mg L−1 and 2.37–2.80 mg L−1. The TN and NH4+-N concentrations of the FBR were considerably lower than those of the PFR.Fig. 2(c) shows that the concentration of NO2−-N in the FBR was reduced slightly from 0.18–0.21 mg L−1 to 0.16 mg L−1, but the concentration of NO2−-N in the PFR increased significantly, from 0.18–0.21 mg L−1 to 0.47–0.56 mg L−1 during the experiment. Fig. 2(d) shows that the concentrations of NO3−-N of FBR and PFR both declined, the initial concentration of NO3−-N was 4.07–5.07 mg L−1, the final NO3−-N concentrations were 1.53–1.63 mg L−1 for FBR and 2.40–2.67 mg L−1 for PFR. There were significant differences between the FBR and PFR in terms of the removal NO3−-N, with the downward trend of NO3−-N being slightly more obvious in the FBR.
Data presented in Fig. 2 indicates that nitrogenous compounds removal efficiency of the FBR was much higher than that of the PFR. The concentration of nitrogenous compounds, as expressed in the concentrations of TN, NH4+-N, NO2−-N and NO3−-N in the FBR, were reduced considerably. In terms of the concentrations of TN, NH4+-N, NO3−-N, NO2−-N, CODMn, statistically significant differences between the FBR and the PFR (p < 0.05) were noted. Compared with the FBR, the concentration of N (as indicated by concentration levels of TN, NH4+-N and NO3−-N in the PFR) decreased slowly. Moreover, the NO2−-N concentration increased slightly. The main reasons why the nitrogenous compounds removal efficiency of FBR was higher than that of the PFR are outlined below.
1. NH4+-N. Due to filamentous bamboo being a natural bio-carrier, a higher bio-affinity and a lower bio-toxicity were the main reasons for the presence of a higher biomass of nitrifying bacteria on this substrate.24,25 This meant that the concentration of NH4+-N declined at a higher rate in the FBR than was the case for the PFR, when maintained under similar conditions.
2. TN. In contrast to the situation pertaining to the plastic filling in the PFR, the filamentous bamboo in the FBR was broken up into soluble matter by bacteria on the surface and then utilized for de-nitrification, resulting in a significantly higher rate of decrease of TN.13,26 Compared to other inert bio-carriers, filamentous bamboo can be decomposed during water treatment, resulting in a thicker biofilm on the bamboo.26 This thicker biofilm provides anaerobic conditions and a sufficient carbon source for the de-nitrification process. Thus the inner biofilm is anoxic, while the outer biofilm layer is aerobic. The depth of the oxic zone depends on the oxygen supply and depletion rates.27 Nitrification therefore takes place at the filamentous bamboo interface, which is an aerobic layer, whereas anoxic micro-zones exist in the deeper layer of the biofilm, which allows heterotrophic denitrifiers to produce nitrogen gas. This contrasts with the situation in the PFR where the anaerobic conditions and de-nitrification carbon source for PFR do not meet demands for treating TN.
3. NO2−-N and NO3−-N. The final NO2−-N and NO3−-N concentrations associated with the FBR were 0.16 mg L−1 and 1.53–1.63 mg L−1, respectively, which were significantly lower than those associated with the PFR, where the final concentrations were 0.47–0.56 mg L−1 and 2.40–2.67 mg L−1, respectively. A high amount of NH4+-N was transformed into NO2−-N and NO3−-N, so the concentrations of the latter two compounds increased slightly. This study also indicated that there were variations in the final NO2−-N and NO3−-N contents between the FBR and PFR, with the removal rates of NO2−-N and NO3−-N in the FBR being higher than those associated with the PFR, because of the use (in the FBR) of filamentous bamboo as a carbon source for de-nitrification. When the NH4+-N was completely oxidized into the NO2−-N and NO3−-N, and the NO2−-N and NO3−-N was simultaneous removal as de-nitrification became stronger.
Effects of two materials on Chl-a removal efficiency
Chl-a is an important index of phytoplankton concentration and an index of eutrophication.3 Some studies have indicated that high levels of algae-lysing bacteria (Pseudomonas sp. and Bacillus sp.) were present on the bio-carriers, with densities of these two microorganisms respectively reaching 3.4 × 1010 and 5.5 × 1010 cells g−1 in the medium.3,28 The Chl-a concentrations were as follows: in raw water, range 83.7–111.6 μg L−1, average 102.3 μg L−1; in the final treated waters of the FBR, range 32–55.8 μg L−1, average 42.6 μg L−1; and in the final treated waters of the PFR, range 74–94.1 μg L−1, average 86.7 μg L−1. This resulted in a mean removal efficiency of 58.36% and 15.25% when using the FBR and the PFR, respectively. The main mechanisms responsible for the reduction of algae using bio-reactors can be described as follows: (1) degradation by enriched algae-lysing bacteria attached to the bio-films; (2) algal growth limitation due to lower nitrogenous compounds concentrations, the removal of nitrogenous compounds by bio-reactors. Compared with the PFR, the FBR has a greater elimination effect on Chl-a removal efficiency. On the other hand, the FBR has a greater elimination capability effect on nitrogenous compounds and a greater biomass due to the presence of filamentous bamboo, which results in a significant difference (p < 0.05), in terms of Chl-a removal efficiency, between the performance of the FBR compared to that of the PFR.Glucose compared with bamboo for de-nitrification
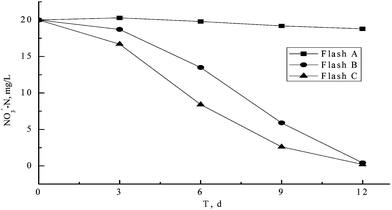
As explained in Section 3.4, in order to confirm the feasibility and efficiency of de-nitrification by filamentous bamboo, the use of glucose, as a substrate for de-nitrification, was compared to that of bamboo.Fig. 3 shows the effect of carbon source on NO3−-N removal. It was obvious that carbon source plays an important role in removing NO3−-N, with results indicating that very little NO3−-N was removed from Flask A in which no extra carbon source was provided, while the NO3−-N was removed fully when carbon source added according to Fig. 3. Filamentous bamboo had the same effect as glucose, in terms of facilitating the removal of NO3−-N, and this effect was enhanced (Fig. 3) resulting in an almost-complete removal of NO3−-N within 12 h. Based on results illustrated in Fig. 3, NO3−-N removal rates could reach levels of 2.09 mg NO3−-N h−1 in the presence of filamentous bamboo, and 2.01 mg NO3−-N h−1 in the presence of glucose.
The relevant statistics show that the NO2−-N accumulated quantity for Flask A, B and C were respectively 68.04 mg, 1.40 mg, and 6.52 mg respectively when the removal of 1 g NO3−-N was found.
Discussion
Based on the bacterial performance in the biofilm, results indicate that nitrifying bacteria, ammonia-oxidizing bacteria and nitrite-oxidizing bacteria can grow at the base of the biofilm where oxygen exists, but heterotrophic de-nitrification bacteria dominate the region adjacent to the bulk liquid, where oxygen is depleted.29–31 The nitrifying bacteria in the aerobic region therefore consumes the oxygen that is available on the biofilm surface. On the plastic filling, however, the heterotrophic de-nitrification bacteria are accumulated at the inner biofilm. Because of the decreased effective diffusion coefficient, the DO, CODMn and NH4+-N cannot be transported into the biofilm via diffusion. Thus the adhesion between the biofilm and the biocarrier is reduced and the biofilm falls from the plastic-filling surface, resulting in low biofilm quantity, low bacterial density, low de-nitrification efficacy and low CODMn, Chl-a removal rates.In contrast to the situation associated with the plastic filling, the heterotrophic de-nitrification bacteria on the filamentous bamboo obtained a relatively sufficient carbon source from the product of bamboo cellulose hydrolysis in the inner biofilm. The biofilm was then able to adhere firmly to the surface on the filamentous bamboo, due to the relative constancy of the microbial population, density, biofilm thickness and biofilm quantity. This resulted in high pollutant bioremediation efficacy, particularly the high TN removal efficacy. The heterotrophic de-nitrification bacteria consumed NO3−-N as an electron donor and also made use of the carbon source from bamboo. The organic matters in the raw water also acted as an electron donor at the inner biofilm region. All these factors led to stable de-nitrification.
Conclusions
The bioremediation of eutrophic river water, using filamentous bamboo and plastic filling as bio-film carriers, was found to be feasible. The average removal rates of TN, NH4+-N, NO3−-N, NO2−-N, CODMn, Chl-a were 63.86%, 47.80%, 64.75%, 20.00%, 63.50% and 58.36% for FBR, and 11.29%, 18.24%, 43.90%, −165%, 9.56% and 15.25% for PFR, respectively. The results showed that the NO2−-N accumulation phenomenon occurred in the PFR. In terms of TN, NH4+-N, NO3−-N, NO2−-N, CODMn and Chl-a, there were statistically-significant differences between the concentrations of these compounds associated with the FBR and PFR (p < 0.05).Our results have shown that eutrophic river water containing refractory organic matter and high nitrogenous compounds can be bio-remediated using biofilm processes. In comparison with results obtained when using inert bio-carriers, the filamentous bamboo is suitable for the formation of a more diverse microbial community and a higher biomass on the surface, as well as providing a more abundant carbon source for de-nitrification, and the carbon source from the product of bamboo cellulose hydrolysis in the inner biofilm. This resulted in higher pollutant removal efficiency (in terms of CODMn, TN and NH4+-N, Chl-a) as well as lower concentrations of NO2−-N. Filamentous bamboo is a potential carbon source for de-nitrification, which could, in the future, compete with glucose as a carbon source for de-nitrification.
Bioremediation of nitrogenous compounds, CODMn and Chl-a from eutrophic surface waters, using biofilms on filamentous bamboo, can therefore be considered as an attractive alternative method.
Acknowledgements
This study was supported by State Key Laboratory of Pollution Control and Resource Reuse Foundation (no. PCRRF13021), Six Talent Summit of Jiangsu Province (no. JNHB-005), Qinglan Project of Jiangsu Province, Youth Fund of Xuzhou Institute of Technology (XKY2011213) and National Spark Program of China (2013GA690421).References
- F. L. Zhao, W. D. Yang and Z. Zeng, Biomass Bioenergy, 2012, 42, 212–218 CrossRef CAS PubMed.
- L. D. Zhu, Z. H. Li and T. Ketola, Ecol. Eng., 2011, 37, 1460–1466 CrossRef PubMed.
- X. N. Li, H. L. Song and W. Li, Ecol. Eng., 2010, 36, 382–390 CrossRef PubMed.
- M. Rodgers and X. M. Zhan, Rev. Environ. Sci. Biotechnol., 2003, 2, 213–224 CrossRef CAS.
- W. P. Cao, J. Xuzhou Inst. Technol., Nat. Sci. Ed., 2012, 27, 73–77 CAS.
- W. P. Cao and S. C. Tan, Chinese J. Environ. Eng., 2010, 4, 1585–1590 CAS.
- B. Ovez, Process Biochem., 2006, 41, 1289–1295 CrossRef CAS PubMed.
- Ş. Aslan and A. Türkman, Water Sci. Technol., 2003, 48, 489–495 Search PubMed.
- A. Boley, J. Mergaert and C. Muller, Acta Hydrochim. Hydrobiol., 2003, 31, 195–203 CrossRef CAS.
- R. S. Alvarez, R. B. Cardoso, M. Szlazar, J. Gómez, E. R. Flores and J. A. Field, Water Res., 2007, 41, 1253–1262 CrossRef PubMed.
- Z. Q. Shen, Y. X. Zhou and J. L. Wang, Bioresour. Technol., 2013, 131, 33–39 CrossRef CAS PubMed.
- Z. Q. Shen and J. L. Wang, Bioresour. Technol., 2011, 102, 8835–8838 CrossRef CAS PubMed.
- W. P. Cao, Y. M. Zhang, Y. F. Li, Y. F. Guo and G. H. Zhang, China Environ. Sci., 2010, 30(8), 328–332 Search PubMed.
- State Environmental Protection Administration of China, Water and wastewater monitor technologies, China Environmental Science Press, Beijing, 2002, P. R. China.
- W. P. Cao, H. H. Zhang, Y. M. Wang and J. Z. Pan, Ecol. Eng., 2012, 42, 146–149 CrossRef PubMed.
- A. Tanyolac and H. Beyenal, Biochem. Eng. J., 1998, 2, 207–216 CrossRef CAS.
- S. Seker, H. Beyenal and A. Tanyolac, J. Biotechnol., 1995, 41, 39–47 CrossRef CAS.
- X. C. Quan, H. C. Shi, Y. M. Zhang, J. L. Wang and Y. Qian, Process Biochem., 2003, 38, 1545–1551 CrossRef CAS.
- E. Karatan and P. Watnick, Microbiol. Mol. Biol. Rev., 2009, 73(2), 310–347 CrossRef CAS PubMed.
- D. B. Schlisselberg and S. Yaron, Food Microbiol., 2013, 35, 65–72 CrossRef CAS PubMed.
- Q. L. Zhao, S. Y. Liu and K. Wang, J. Harbin Univ. Civ. Eng. Archit., 1999, 32(6), 39–43 Search PubMed.
- Z. S. Lin, Acta Ecol. Sin., 2002, 22(4), 535–540 Search PubMed.
- S. Cousins, Oikos, 1990, 57(2), 270–275 CrossRef.
- C. D. Nadell, J. B. Xavier and K. R. Foster, FEMS Microbiol. Rev., 2009, 33(1), 206–224 CrossRef CAS PubMed.
- L. R. Hoffman, D. A. Dargenio, M. J. MacCoss, Z. Zhang, R. A. Jones and S. I. Miller, Nature, 2005, 436(7054), 1171–1175 CrossRef CAS PubMed.
- W. P. Cao, Water Environ. Res., 2014, 102, 456 Search PubMed.
- S. Yang, F. L. Yang, Z. M. Fu and R. B. Lei, Bioresour. Technol., 2009, 100, 2369–2374 CrossRef CAS PubMed.
- R. P. Ji, X. W. Lu, X. N. Li and Y. P. Pu, Ecol.Eng., 2009, 35, 1584–1588 CrossRef PubMed.
- A. C. Cole, M. J. Semmens and T. M. LaPara, Appl. Environ. Microbiol., 2004, 70, 1982–1989 CrossRef CAS PubMed.
- K. Hibiya, A. Terada, S. Tsuneda and A. Hirata, J. Biotechnol., 2003, 100, 23–32 CrossRef CAS.
- Y. Q. Zhang, W. P. Cao, L. Liu, X. R. Han and H. Huang, J. Xuzhou Inst. Technol., Nat. Sci. Ed., 2013, 28, 20–25 CAS.
| This journal is © The Royal Society of Chemistry 2014 |