Chemiresistive metal-stabilized thiyl radical films as highly selective ethylene sensors†
Rajat Chauhan,
Monica Moreno,
Douglas M. Banda,
Francis P. Zamborini* and
Craig A. Grapperhaus*
Department of Chemistry, University of Louisville, 2320 South Brook Street, Louisville, KY 40292, USA. E-mail: grapperhaus@louisville.edu; f.zamborini@louisville.edu; Tel: +1-502-852-5932
First published on 16th September 2014
Abstract
A highly selective chemiresistive ethylene sensor based on reversible and selective ligand-centered substrate binding to a metal-stabilized thiyl radical has been developed. The solid-state device efficiently differentiates between ethylene and other alkene analytes. The sensor is prepared by simple dropcast deposition of the complex as a film across a microgap gold electrode.
Naturally evolved ethylene gas is responsible for the large-scale spoilage of agricultural products due to its role as a plant hormone involved in such processes as fruit ripening, germination, and leaf abscission.1,2 During transportation/storage, ethylene levels as low as 10 ppb can cause product loss.3 As such, there is a need for simple and portable gas-phase ethylene detectors to complement traditional ethylene detection methods that require laboratory analysis of collected air samples by gas chromatography4–6 or photoacoustic spectroscopy.7
Recent advances in ethylene detection include a reversible chemiresistor comprised of Pt complexes and Au nanoparticles with ppb detection limits, but with poor selectivity due to the nature of the interaction of the substrate with the Pt metal.8 The direct coordination of ethylene and other small molecules including alkenes at the metal-center precludes a differential response for ethylene. Similar selectivity issues were observed for metal-oxide based resistors9–13 and metal-based photoluminescence14,15 and colorimetric16 detectors. To overcome the selectivity problem, herein we report a chemiresistive ethylene sensor based on the ligand-centered addition of ethylene to the metal-stabilized thiyl radical complex [Re-1]+ (Scheme 1) which has been previously reported to display (1) kinetic selectivity for ethylene binding over other alkenes, alkynes, and dienes;17,18 (2) inertness towards H2, alkanes, and other saturated analytes;17 and (3) quick and reversible ethylene binding.19
To translate the solution reactivity of [Re-1]+ to a solid substrate, a film of the metal complex was deposited by dropcast across a microgap electrode, Fig. 1. Two Au electrodes separated by 23 μm were fabricated in a clean room facility by photolithography on a Si/SiOx substrate, Fig. S1,† using previously described methods.20–22 Wire leads were attached to the Au contact pads with Ag epoxy, which was further insulated with an overlayer of Torr-seal epoxy. The electrodes were cleaned by rinsing in acetone, ethanol, and 2-propanol before drying under N2. The device was then placed in a UVO ozone cleaner for 10 min prior to film deposition.
 | ||
| Fig. 1 Schematic of [Re-1]+ coated Au microgap electrodes showing the metal-stabilized thiyl radical film (blue circles) deposited across a 23 μm gap between the gold electrodes E1 and E2. | ||
A 3 mM [Re-1]+ complex solution was prepared by oxidation of [Re-1] with ferrocenium hexafluorophosphate in dichloromethane inside a nitrogen filled glovebox. The deep blue solution of [Re-1]+ was transferred dropwise to the electrode surface and allowed to evaporate forming a thin film. The radical complex [Re-1]+ film bridges the gap between electrodes E1 and E2 forming an ethylene sensitive resistor. The modified electrode was transferred to a gas mixing chamber with ethylene and nitrogen supplies (Fig. S2†). A bias of 1 V was applied and the current measured in the presence of variable ethylene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nitrogen ratios under ambient conditions.
nitrogen ratios under ambient conditions.
Exposure of the [Re-1]+ modified electrode to 100% ethylene with an applied bias of 1 V results in a significant decrease in current (Fig. 2). The mode of detection is attributed to an electron hopping mechanism in the delocalized metal-stabilized thiyl radical [Re-1]+ that is hindered in the presence of ethylene due to localization of the electron spin on the metal-center in the analyte bound complex [Re-1·C2H4]+.17 The current is restored when nitrogen is reintroduced. Response time for ethylene detection is less than 10 seconds. Refreshing the electrode with a nitrogen purge requires 100 seconds consistent with the relative values of the rate constants for ethylene addition (kf = 0.12(2) M−1 s−1) and ethylene release (kr = 0.030(4) s−1) measured in solution.19 Repeated cycling of 100% ethylene and 100% nitrogen confirms the reversibility of ethylene binding and reproducibility of the current response.
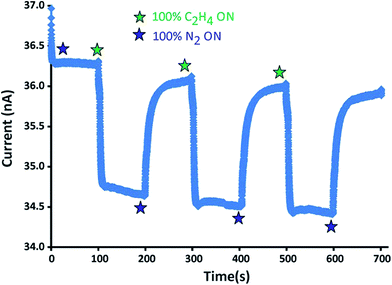 | ||
| Fig. 2 Plot of current (nA) versus time (s) for the solid-state [Re-1]+ coated Au microgap electrodes with cycling of gaseous C2H4 (green star) and N2 (blue star). | ||
For ethylene concentrations between 0.9% and 31.3%, the average percent response of 3–5 trials is 0.41 to 3.52 with standard deviations of 0.06–0.39, Fig. 3 and 4, Table 1, and Fig. S3–S8.† Data plotted is for a single sensor, with similar results obtained with other devices. The solid state data shows a rapid increase in percent response at low ethylene concentrations allowing quantification of gaseous concentrations between 0.4 and 20%. At higher ethylene concentrations, the binding sites within the film become saturated and the response begins to plateau near 30% ethylene with only gradual signal increases with additional analyte. Response times at low ethylene concentrations are significantly longer, up to 1000 seconds, which may be related to the rate constant for ethylene addition and/or film reproducibility. Efforts are underway to lower the limit of detection, which is relatively high compared to other ethylene chemiresistors.
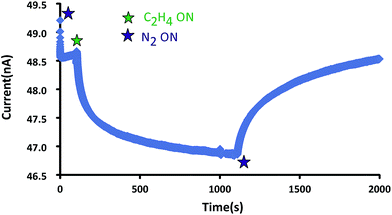 | ||
| Fig. 3 Plot of current (nA) versus time (s) for the solid-state [Re-1]+ coated Au microgap electrodes in the presence of gaseous 31.3% C2H4. | ||
 | ||
| Fig. 4 Plot of current response percent versus gaseous C2H4 concentration (0.8% to 31.2%) for solid-state [Re-1]+ coated microelectrodes. | ||
| Percent ethylene | Percent responsea | Standard deviation |
|---|---|---|
| a Percent response calculated as 100 × (iN − iE)/iN where iN = current in the presence of N2 and iE = current in the presence of C2H4. Reported value are for an average 3–5 trials using a single sensor. | ||
| 0 | 0 | 0 |
| 0.9 | 0.41 | 0.06 |
| 2.8 | 1.39 | 0.44 |
| 4.0 | 1.49 | 0.15 |
| 11.1 | 2.49 | 0.14 |
| 19.4 | 3.35 | 0.19 |
| 26.3 | 3.11 | 0.22 |
| 31.2 | 3.52 | 0.40 |
A significant advantage of the current system is its high selectivity. The selectivity of the sensor derives from the steric constraint of the ligand-centered ethylene binding site. Prior solution studies revealed kinetically retarded binding of larger alkenes to [Re-1]+ and no observable binding of H2, CO, or other small molecules.17,18 To demonstrate the selectivity of the solid state device, the detector was exposed to a gaseous mixture of 19.3% 1-hexene and nitrogen. No detectable change in resistivity was observed, whereas the same concentration of ethylene provided a response of over 3.5%, Fig. 5. Additionally, the electrodes are air-stable and can be stored for up to 6 months under ambient conditions with no significant change in response to 100% ethylene despite the air-sensitivity of [Re-1]n in solution.
 | ||
| Fig. 5 Plot of normalized current versus time (s) for solid-state [Re-1]+ coated Au microgap electrodes in the presence of gaseous 19.3% ethylene (blue) and gaseous 19.3% 1-hexene (red) at 19.5 °C. | ||
A comparison of the solid-state and solution sensing data confirms that we have successfully translated the solution reactivity of our metal-stabilized thiyl radical to a heterogeneous system. Solution detection data were collected via square wave voltammetry with an initial potential of +168 mV versus ferrocenium/ferrocene, Fig. S10.† Under N2, a single cathodic event is observed at −345 mV associated with the [Re-1]+/0.19 When the same conditions were employed under an ethylene atmosphere, an additional cathodic peak at −100 mV corresponding to [Re-1·C2H4]2+/+ was observed with a relative peak intensity equal to 50% of the remaining [Re-1]+/0 peak. Data collected with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of ethylene
1 ratio of ethylene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nitrogen yielded the same two cathodic peaks with a significant decrease in the relative intensity of the [Re-1·C2H4]2+/+ couple. Varying the initial hold-time did not significantly alter the ratio of two cathodic events. This potential was held for 120 s during which time Re-1 is oxidized to [Re-1]+. The potential was then scanned to a final value of −732 mV. Full experimental details are provided in the ESI.†
nitrogen yielded the same two cathodic peaks with a significant decrease in the relative intensity of the [Re-1·C2H4]2+/+ couple. Varying the initial hold-time did not significantly alter the ratio of two cathodic events. This potential was held for 120 s during which time Re-1 is oxidized to [Re-1]+. The potential was then scanned to a final value of −732 mV. Full experimental details are provided in the ESI.†
Multiple square wave voltammograms were collected at various ethylene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nitrogen ratios and each measurement was repeated at least three times. Average relative peak intensities were plotted versus percent ethylene, Fig. 6. The curve shows a sigmoidal pattern with no significant binding detected below 30% ethylene and saturation binding above 70%. The relatively high limit of detection is attributed to the weak ethylene binding affinity, K2 = 4 M−1, of the [Re-1]+ complex.
nitrogen ratios and each measurement was repeated at least three times. Average relative peak intensities were plotted versus percent ethylene, Fig. 6. The curve shows a sigmoidal pattern with no significant binding detected below 30% ethylene and saturation binding above 70%. The relatively high limit of detection is attributed to the weak ethylene binding affinity, K2 = 4 M−1, of the [Re-1]+ complex.
 | ||
| Fig. 6 Plot of relative area of the [Re-1·C2H4]2+/+ couple in the square wave voltammogram of [Re-1] in CH2Cl2 solution as a function of percent C2H4 purged through the solution. | ||
Conclusions
Using simple dropcast methods, a highly selective chemiresistive device has been constructed that allows for the reversible detection of gaseous ethylene. The mode of detection is attributed to an electron hopping mechanism in the delocalized metal-stabilized thiyl radical that is hindered in the presence of ethylene due to localization of the electron spin on the metal-center. Similar selectivity between the heterogeneous and homogenous phase suggests that the mode of binding is similar with a sterically restricted ligand-centered binding site responsible for the high selectivity. The selective and reversible nature of this system is unique. The electrodes are simple to construct, long-lived, and functional under ambient conditions. Improving sensitivity remains a challenge and efforts to construct a device capable of sub-ppm level ethylene sensing based on the stronger binding of [Re-1]2+, which has an equilibrium binding constant of 2.5 × 109 M−1,19 are underway.Acknowledgements
Acknowledgment is made to the Donors of the American Chemical Society Petroleum Research Fund (51566-ND3) for support of this work. DMB acknowledges financial support from the Office of the Executive Vice President for Research and Innovation at the University of Louisville through the Summer Research Opportunity Program.Notes and references
- S. F. Yang and N. E. Hoffman, Annu. Rev. Plant Physiol., 1984, 35, 155–189 CrossRef CAS.
- R. B. H. Wills, M. A. Warton and V. V. V. Ku, Aust. J. Exp. Agric., 2000, 40, 465–470 CrossRef CAS.
- G. M. Casciano, J. Moore, M. Rexrode, D. Benmhend and P. Ross, United States Environmental Protection Agency Report EPA-HQ-OPP-2009-0877, 2009 Search PubMed.
- H. Pham-Tuan, J. Vercammen, C. Devos and P. Sandra, J. Chromatogr. A, 2000, 868, 249–259 CrossRef CAS.
- L. Eklund and C. H. A. Little, Tree Physiol., 1998, 18, 383–391 CrossRef CAS.
- A. Sklorz, S. Janssen and W. Lang, Sens. Actuators, B, 2013, 180, 43–49 CrossRef CAS PubMed.
- H. S. M. deVries, M. A. J. Wasono, F. J. M. Harren, E. J. Woltering, H. C. P. M. vanderValk and J. Reuss, Postharvest Biol. Technol., 1996, 8, 1–10 CrossRef CAS.
- M. P. Rowe, W. H. Steinecker and E. T. Zeller, Anal. Chem., 2007, 79, 1164–11172 CrossRef CAS PubMed.
- M. Agarwal, M. D. Balachandran, S. Shrestha and K. Varahramyan, J. Nanomater., 2012, 2012, 5 Search PubMed.
- P. Ivanov, E. Llobet, A. Vergara, M. Stankova, X. Vilanova, J. Hubalek, I. Gracia, C. Cané and X. Correig, Sens. Actuators, B, 2005, 111–112, 63–70 CrossRef CAS PubMed.
- S. Pitcher, J. A. Thiele, H. Ren and J. F. Vetelino, Sens. Actuators, B, 2003, 93, 454–462 CrossRef CAS.
- R. Zhang, M. I. Tejedor, M. A. Anderson, M. Paulose and C. A. Grimes, Sensors, 2002, 2, 331–338 CrossRef CAS PubMed.
- B. S. Kang, S. Kim, F. Ren, K. Ip, Y. W. Heo, B. Gila, C. R. Abernathy, D. P. Norton and S. J. Pearton, J. Electrochem. Soc., 2004, 151, G468–G471 CrossRef CAS PubMed.
- M. Santiago Cintrón, O. Green and J. N. Burstyn, Inorg. Chem., 2012, 51, 2737–2746 CrossRef PubMed.
- O. Green, N. A. Smith, A. B. Ellis and J. N. Burstyn, J. Am. Chem. Soc., 2004, 126, 5952–5953 CrossRef CAS PubMed.
- P. Cabanillas-Galán, L. Farmer, T. Hagan, M. Nieuwenhuyzen, S. L. James and M. C. Lagunas, Inorg. Chem., 2008, 47, 9035–9041 CrossRef PubMed.
- K. Ouch, M. S. Mashuta and C. A. Grapperhaus, Inorg. Chem., 2011, 9904–9914 CrossRef CAS PubMed.
- K. Ouch, M. S. Mashuta and C. A. Grapperhaus, Eur. J. Inorg. Chem., 2012, 2012, 475–478 CrossRef CAS.
- C. A. Grapperhaus, K. Ouch and M. S. Mashuta, J. Am. Chem. Soc., 2009, 131, 64–65 CrossRef CAS PubMed.
- R. Dasari and F. P. Zamborini, J. Am. Chem. Soc., 2008, 130, 16138–16139 CrossRef CAS PubMed.
- F. J. Ibanez and F. P. Zamborini, Langmuir, 2006, 22, 9789–9796 CrossRef CAS PubMed.
- F. J. Ibanez and F. P. Zamborini, ACS Nano, 2008, 2, 1543–1552 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental methods including device preparation, detection methodology, and experimental setup. See DOI: 10.1039/c4ra07560a |
| This journal is © The Royal Society of Chemistry 2014 |

