Fe4[Fe(CN)6]3: a cathode material for sodium-ion batteries†
He Sun,
Haobo Sun,
Wei Wang,
Handong Jiao and
Shuqiang Jiao*
State Key Laboratory of Advanced Metallurgy, University of Science and Technology Beijing, Xueyuan Road 30, Beijing 100083, P. R. China. E-mail: sjiao@ustb.edu.cn; Fax: +86-10-62334204
First published on 28th August 2014
Abstract
Insoluble Prussian blue (IPB), Fe4[Fe(CN)6]3, is synthesized at room temperature and found to achieve a relatively high storage capacity of 146 mA h g−1 (at a 20 mA g−1 rate) as the cathode material for Na-ion batteries with high potential plateaus. XRD, SEM and TEM are employed to characterize the morphology and microstructure of the as-synthesized IPB. First-principles calculation is introduced to study the electrochemical mechanism. Generally, the environmental friendliness and low cost of the material make it possible to be used for large-scale electric storage applications.
1. Introduction
Lithium ion batteries (LIBs) now play an important role in large-scale electric energy storage (EES), and the use of LIBs in portable devices and electric vehicles has greatly expanded. Considering the extensive future application of LIBs along with the uneven distribution of lithium sources, which are mainly located in South America, the cost of lithium will become a critical issue for future LIBs.1 Thus, the availability of lithium will be limited, and its price will increase considerably in the near future.2 Compared to LIBs, sodium-ion batteries (SIBs) are potentially cheaper due to the low cost and abundance of sodium in the earth, making SIBs suitable for large scale energy storage devices in which high energy density is less critical.3Over the past few decades, considerable academic interest in SIBs has been focused on the development of cathode materials. Metal hexacyanoferrates have been used as cathodes for SIBs because of their possible alkali metal cation storage capabilities.4 The prototype of these metal hexacyanoferrates is Prussian blue (KM[Fe(CN)6], M = Mn, Fe, Co, Ni, Cu…), which has a fairly open cubic framework with large interstitial sites able to easily accommodate compensating counter cations during redox reactions.5 Presently, with the wider study of Prussian blue structures as electrode materials, two kinds of Prussian blue (soluble Prussian blue (SPB; KFeFe(CN)6) and insoluble Prussian blue (IPB; Fe4[Fe(CN)6]3), have been found to possess good electrochemical properties.6 Y. Cao et al. reported a nanosized Na4Fe(CN)6/C composite as an SIB cathode material that showed stable charge/discharge plateaus and a realized discharge capacity of 87 mA h g−1 at 0.1 C (9 mA g−1); for comparison, the theoretical one-electron transfer capacity is 89 mA h g−1.7 J. B. Goodenough and co-workers introduced a structure of SPB and its analogues with a cubic framework (space group Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m), with Fe(II) and Fe(III) on alternate corners of a cube of corner-shared octahedra bridged by linear C
m), with Fe(II) and Fe(III) on alternate corners of a cube of corner-shared octahedra bridged by linear C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N−; the low-spin Fe(III) bonds only with C atoms, the high-spin Fe(II) bonds only with N atoms, and the C
N−; the low-spin Fe(III) bonds only with C atoms, the high-spin Fe(II) bonds only with N atoms, and the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N− bond opens the faces of the elementary cubes, allowing Na+ to move between the half-filled body-centre positions.8 This SPB-cathode Na-ion battery exhibits a capacity of ∼100 mA h g−1, with two high potential plateaus of ∼3.5 V and ∼2.8 V.
N− bond opens the faces of the elementary cubes, allowing Na+ to move between the half-filled body-centre positions.8 This SPB-cathode Na-ion battery exhibits a capacity of ∼100 mA h g−1, with two high potential plateaus of ∼3.5 V and ∼2.8 V.
2. Experimental section
2.1 Materials
In this work, Fe4[Fe(CN)6]3 was synthesized by a one-step liquid reaction at room temperature following the overall reaction below:| 4FeCl3 + 3Na4Fe(CN)6 → Fe4[Fe(CN)6]3 + 12NaCl | (1) |
FeCl3 and Na4Fe(CN)6·10H2O were purchased from Aladdin reagent company. Before use, the reagents were ground in an agate mortar for 30 minutes. A 100 mM FeCl3 solution was prepared by adding 50 ml of deionised water into 5 mmol FeCl3. The solution was stirred for 10 minutes so as to be completely dissolved. The 75 mM Na4Fe(CN)6 solution was obtained in the same manner by adding 50 ml of deionized water into 3.75 mmol Na4Fe(CN)6. The Na4Fe(CN)6 solution was then added slowly into the FeCl3 solution; a dark blue precipitate appeared immediately. After 20 minutes of magnetic stirring at 55 °C, the precipitate was obtained by centrifuging five times, each for 10–30 minutes. The Fe4[Fe(CN)6]3 product was placed in a 60 °C constant temperature oven to dry overnight.
The electrode was prepared by mixing the synthesized Fe4[Fe(CN)6]3 with acetylene black and Teflon (polytetrafluoroethylene, PTFE) binder at a weight ratio of 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 to form a homogeneous slurry. After drying in an oven at 60 °C for several hours, the slurry was painted as thinly as possible onto aluminium foil and then dried at 120 °C for at least 12 hours. The electrolyte was 1.0 M NaClO4 in propylene carbonate (PC) solution. The glass fibre (GF/D) separator was purchased from Whatman. Electrochemical measurements were conducted at room temperature in coin cells prepared inside an Ar-filled glove box.
10 to form a homogeneous slurry. After drying in an oven at 60 °C for several hours, the slurry was painted as thinly as possible onto aluminium foil and then dried at 120 °C for at least 12 hours. The electrolyte was 1.0 M NaClO4 in propylene carbonate (PC) solution. The glass fibre (GF/D) separator was purchased from Whatman. Electrochemical measurements were conducted at room temperature in coin cells prepared inside an Ar-filled glove box.
2.2 Structural characterizations
The morphology and microstructure of the as-prepared sample were characterized by X-ray diffraction (XRD, Rigaku D/max-RB), scanning electron microscopy (SEM, JEOL JSM-6480LV) and transmission electron microscopy (TEM JEOL, JEM-2010).The first-principles plane-wave pseudopotential method was implemented in CASTEP, which is used for calculating the formation energy of the unit cell volume change for Na-ion insertion. This method was introduced to illustrate the change in crystal structure and the insertion–extraction mechanism of Na ions in Fe4[Fe(CN)6]3.9,10 The generalized gradient approximation (GGA) with norm-conserving pseudopotentials was used with a plane-wave cut-off of 370 eV.11 Brillouine-zone integrations were performed using (3 × 3 × 3) special-k-point meshes according to the Monkhorst–Pack scheme.12 For each geometry optimization, both atomic positions along with the lattice parameters were fully relaxed using the quasi-Newton method.13 The convergence thresholds between optimization cycles for energy change, maximum force, maximum stress, and maximum displacement were set as 1 × 10−5 eV per atom, 0.03 eV Å−1, 0.05 GPa, and 0.001 Å, respectively. The optimization was terminated when all of these criteria were satisfied. The choice of these computational parameters ensured good convergence in the present work.
2.3 Thermogravimetry analysis experiments
Thermogravimetry analysis (TGA, NETZSCH, STA409C) was conducted in the temperature range of 30 °C to 300 °C to calculate the number of H2O molecules contained in Fe4[Fe(CN)6]3.2.4 Electrochemical tests
Galvanostatic discharge/charge tests were implemented using the Neware BTS-53 tester. The cut-off voltage was set in the range of 1.5 V to 4.0 V vs. Na/Na+. Cyclic voltammograms (CV, CHI 1140A) were measured from 1.5 V to 4.0 V at a scan rate of 0.1 mV s−1. Electrochemical impedance spectroscopy (EIS, CHI 1140A) was employed to investigate the detailed reaction kinetics of the as-prepared electrode. The frequency range was 1.0 × 10−5 Hz to 0.1 Hz at an amplitude of 5 mV.3. Results and discussion
The XRD pattern (Fig 1a) shows that all Bragg peaks are well indexed to the pure phase of Fe4[Fe(CN)6]3 (JCPDS file number 73-687).14 The SEM image (Fig. S1†) shows small, relatively equal-sized particles of Fe4[Fe(CN)6]3. However, agglomeration resulting from the use of the hydrothermal method is obvious and inevitable. Further characterization by TEM is displayed in Fig. 1b; the microstructure of the as-synthesized Fe4[Fe(CN)6]3 had an average size of 50–70 nm. | ||
| Fig. 1 (a) XRD pattern and (b) TEM images of the synthesized Fe4[Fe(CN)6]3 and (c) framework of Prussian blue. | ||
H. J. Buser et al. reported a non-face-centred structure for Prussian blue, which deviates from the model of cubic polynuclear transition-metal cyanides of the space group Fm3m (Oh5).6 In addition, the Fe(CN)6 positions are only partly occupied. Therefore, considering the defects in the framework, a primitive framework consisting of two Fe8C24N24 molecules was constructed, as shown in Fig. 1c, in which every Fe atom forms an octahedron with C or N atoms around it. In this structure, there are large interstitial sites for the insertion of Na ions; this scenario will be demonstrated in the following sections.
With sodium ions successively intercalating into Fe4[Fe(CN)6]3, the formation energy is defined as:
| Ef(Na) = Etot(NanFe7C18N18) − Etot(Fe7C18N18) − nEtot(Na) | (2) |
Here, Etot(NanFe7C18N18) is the total energy of Fe4[Fe(CN)6]3 containing n sodium atoms in a primitive cell, Etot(Fe7C18N18) is the total energy of Fe4[Fe(CN)6]3 without any other sodium atoms, and Etot(Na) is the total energy of a sodium atom in the reservoir.
The energy calculation data is given in the Table S1.† 14 Because the IPB framework has defects in its structure, the as-constructed Fe8C24N24 was used for calculation instead. As shown in Table S1,† the formation energy of Fe7C24N24–Na(II) is 10.81 eV (>0 eV), indicating that Fe(II) ions cannot be replaced by Na ions. Similarly, with a formation energy of 6.79 eV, Fe(III) ions cannot be replaced by Na ions. In summary, the Na ions are inserted/extracted into/from the IPB instead of replacing the Fe2+ or Fe3+ cations during the charge/discharge process. The same conclusion can be reached from Fig. 2a, which shows the first-principles calculation of the formation energy of Na inserted in Fe4[Fe(CN)6]3. As more Na ions are inserted, the formation energy becomes more negative, indicating that the structure with Na inserted is more stable. Therefore, Na ions can be inserted only into the Prussian blue structure.
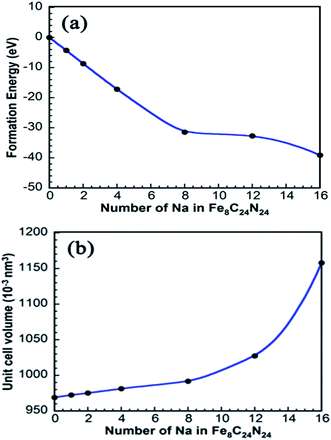 | ||
| Fig. 2 (a) The relationship between the formation energy and the number of Na ions in Fe4[Fe(CN)6]3 and (b) the volume with respect to the number of Na ions in Fe4[Fe(CN)6]3. | ||
The volume change of Fe4[Fe(CN)6]3 with different numbers of inserted sodium ions is displayed in Fig. 2b. The volume of the unit cell increases as the number of Na ions increases. The volume increase does not become significant until the number of Na ions reaches 12.0. When 16.0 Na ions are inserted, the structure collapses immediately; thus, it can be deduced that a maximum of 12.0 Na ions can be inserted into the Fe8C18N18 structure.
From Fig. S2(a),† we find that an obvious mass loss occurred at a temperature of 211.8 °C. However, the mass loss does not stop when the temperature increases, indicating that Fe4[Fe(CN)6]3 partly decomposed. Subsequently, the Fe4[Fe(CN)6]3 was sintered in an Ar atmosphere from 30 °C to 300 °C, the same conditions as the TGA, and a black powder was finally obtained. The black powder was then characterised by XRD (Fig. S2(b)†); the product consisted of Fe4[Fe(CN)6]3 (JCPDS number 73-687) and iron oxides (Fe3O4; JCPDS number 75-1372). Thus, we can infer that Fe4[Fe(CN)6]3 dehydrates below 211.8 °C and decomposes above 211.8 °C. According to the mass loss, we calculated that 12.11 water molecules were contained in Fe4[Fe(CN)6]3.
The galvanostatic charge/discharge curve of a Fe4[Fe(CN)6]3 cathode is demonstrated in Fig. 3a. In the first cycle, the charge capacity is as high as 268 mA h g−1 at a current density of 50 mA g−1, which might be ascribed to some side reactions. The realized discharge capacity is 141 mA h g−1 for the first cycle. Two obvious discharge plateaus are observed: one at ∼3.2 V and the other at ∼2.8 V.
 | ||
| Fig. 3 (a) The 1st, 3rd, 5th, 10th and 50th charge/discharge curves of Fe4[Fe(CN)6]3 at 50 mA g−1 and (b) CV curves of the synthesized Fe4[Fe(CN)6]3. | ||
The CV curves displayed in Fig. 3b show two peaks at 3.03 V and 3.53 V in the charge curve and two peaks at 2.77 V and 3.38 V in the discharge curve of the first cycle. During the charge/discharge process, the following two reactions, which correspond to the two pairs of redox peaks, occurred:15
| Fe4[Fe(CN)6]3 + nNa+ + ne → NanFe4[Fe(CN)6]3 | (3) |
| Fe4[Fe(CN)6]3 + 3A− → 3e + Fe4[Fe(CN)6A]3 | (4) |
For the peak appearing at 3.53V/3.38V, it is considered that A− anions, which are provided by the electrolyte and considered herein as ClO4−, fill in the vacancies of the Fe4[Fe(CN)6]3 to form a more stable structure. However, the inserting process of A− anions is irreversible, which causes the disappearance of the plateau of ~3.2V in the following charge/discharge process.
The cycle performance of the as-prepared Fe4[Fe(CN)6]3 was also investigated by varying the charge/discharge current density from 20 to 100 mA g−1. Generally, the first cycle shows extraordinarily large charge capacity, which is considered to result from some side reactions. In Fig. 4a, the specific discharge capacity at 20 mA g−1 is as high as 146 mA h g−1. Compared to the capacity of the second cycle, Fe4[Fe(CN)6]3 exhibits a capacity retention of 87% at the 25th cycle and 82% at the 50th cycle. Even at a high current density of 100 mA g−1, the battery still performs at a good capacity retention of 90% at the 25th cycle and 86% at the 50th cycle. Thus, there is a stable framework for the intercalation and de-intercalation of Na ions.
 | ||
| Fig. 4 (a) The second charge/discharge curves tested at different current densities and (b) cycling performance under different current densities. | ||
The representative Nyquist plots of the different cycles are shown in Fig. S3†; obvious semicircles attributed to charge/discharge impedance are observed in the high frequency region. In the low frequency region, the slope lines (about 45°) are due to Warburg impedance, which is attributed to the semi-infinite diffusion of Na ions into the electrode–electrolyte interface. In this figure, we observe a decrease in charge/discharge impedance as the cycle number increases. Here, we think that the resistance is at a high level when the battery starts to activate. Later, in the 10th and 50th cycle, the impedance decreases, corresponding to the formation of a more stable Prussian blue framework in which Na ions are easily inserted/extracted.
4. Conclusion
In summary, the as-synthesized insoluble Prussian blue (Fe4[Fe(CN)6]3) exhibits a relatively high storage capacity of 146 mA h g−1 (at 20 mA g−1 rate) with high potential plateaus. First-principles calculations show a feasible way for sodium ions to insert/extract in/from IPB. A good cycle stability with an 82% capacity retention over 50 cycles is also obtained in electrochemical tests, demonstrating the possibility for practical use. In general, the environmental friendliness and low cost of this cathode material make it possible to be used for large-scale electric storage applications.Acknowledgements
This work was supported by the National Basic Research Program of China (no. 2013CB632404).Notes and references
- V. Palomares, P. Serras, I. Villaluenga, K. B. Hueso, J. Carretero-Gonzalez and T. Rojo, Energy Environ. Sci., 2012, 5, 5884 CAS.
- J. M. Tarascon, Nat. Chem., 2010, 2, 510 CrossRef CAS PubMed.
- S. W. Kim, D. H. Seo, X. Ma, G. Ceder and K. Kang, Adv. Energy Mater., 2012, 2, 710–721 CrossRef CAS PubMed.
- A. V. Cmmbliss, P. S. Lugg and N. Morosoff, Inorg. Chem., 1984, 23, 4701 CrossRef.
- M. Giorgetti, M. Berrettoni, A. Filipponi, P. J. Kulesza and R. Marassi, Chem. Phys. Lett., 1997, 275, 108 CrossRef CAS.
- H. J. Buser, D. Schwarzenba, W. Petter and A. Ludi, Inorg. Chem., 1977, 16, 2704 CrossRef CAS.
- J. Qian, M. Zhou, Y. Cao, X. Ai and H. Yang, Adv. Energy Mater., 2012, 2, 410 CrossRef CAS PubMed.
- Y. Lu, L. Wang, J. Cheng and J. B. Goodenough, Chem. Commun., 2012, 48, 6544 RSC.
- M. C. Payne, M. P. Teter, D. C. Allan, T. A. Arias and J. D. Joannopoulos, Rev. Mod. Phys., 1992, 64, 1045 CrossRef CAS.
- S. J. Clark, M. D. Segall, C. J. Pickard, P. J. Hasnip, M. J. Probert, K. Refson and M. C. Payne, Z. Kristallogr., 2005, 220, 567 CrossRef CAS.
- J. S. Lin, A. Qtseish, M. C. Payne and V. Heine, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 47, 4174 CrossRef.
- H. J. Monkhorst and J. D. Pack, Phys. Rev. B: Solid State, 1976, 13, 5188 CrossRef.
- T. H. Fischer and J. Almlof, J. Phys. Chem., 1992, 96, 9768 CrossRef CAS.
- F. Herren, P. Fischer, A. Ludi and W. Hälg, Inorg. Chem., 1980, 19, 956 CrossRef CAS.
- K. Itaya and I. Uchida, Acc. Chem. Res., 1986, 19, 162 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: First-principles calculation data and additional figures. See DOI: 10.1039/c4ra07531e |
| This journal is © The Royal Society of Chemistry 2014 |
