Synthesis and optimization of CdTe quantum dots with the help of erythorbic acid and ethanol
Yan Liangab,
Jiawei Tanab,
Jiexin Wangab,
Jianfeng Chenab,
Baochang Sun*ab and
Lei Shao*ab
aState Key Laboratory of Organic–Inorganic Composites, Beijing University of Chemical Technology, Beijing 100029, China. E-mail: sunbc@mail.buct.edu.cn; shaol@mail.buct.edu.cn; Fax: +86 10 64434784; Tel: +86 10 64421706/64443134
bResearch Center of the Ministry of Education for High Gravity Engineering and Technology, Beijing University of Chemical Technology, Beijing 100029, China
First published on 15th September 2014
Abstract
The effects of erythorbic acid (EA) and ethanol on the aqueous formation of cadmium telluride (CdTe) quantum dots (QDs) were explored in this work. Without N2 protection, CdTe QDs were synthesized with cadmium chloride and sodium hydrogen telluride as the Cd source and Te source, respectively, together with EA and with 3-mercaptopropionic acid (MPA) as the co-passivating ligand. The experimental results indicated that the use of the oxygen scavenger, i.e., EA, was critical for the formation of the CdTe QDs with reasonably good optical properties. Including ethanol during the synthesis improved the photoluminescence intensity. To attain good optical properties, it is also important to tune experimental parameters such as pH, temperature, reaction time, molar ratio of MPA/Cd, and sodium borohydride dosage. The very reason that EA promoted formation of CdTe QDs is because of its reducibility and passivation on the QD surface. The present study suggests that the use of EA and ethanol could be a practical means to promote the photoluminescence of CdTe.
1. Introduction
Nanostructured semiconductors, in particular quantum dots (QDs), are some of the most promising types of nanoparticles that hold potential for a variety of new applications, such as light-emitting devices,1 solar cells,2 and bio-labeling,3 because of their unique optical properties.4–8 Cadmium telluride (CdTe) QDs are the subjects of intense studies and wide applications because they absorb throughout the visible and near-infrared spectra, and display narrow and widely tunable photoluminescence together with a high stability against photo-bleaching when compared with traditional organic dyes.9–18To date, several different methods, using organic and aqueous pathways, have been developed to synthesize CdTe QDs.19–23 Although high quality CdTe QDs can be prepared in the organic phase, they are unable to be used directly in biosystems because of the hydrophobicity of the QDs. Compared with organic synthesis, aqueous synthesis has the advantages of simplicity, high reproducibility, low toxicity, and low cost.24,25 In addition, the aqueous process associated with relatively low temperatures (typically 90–100 °C) is green and much less expensive, which is more attractive for the large scale production of QDs.
In aqueous synthesis of QDs, weak reductive agents such as hydrazine (N2H4) can scavenge oxygen and usually promote aqueous growth of QDs. This simple approach to improve luminescence of CdTe QDs takes advantage of the reducibility of N2H4 to protect QDs and thiol ligands from oxidation.26–28 In our previous study, erythorbic acid (EA), an efficient oxygen scavenger and weak reductive agent, was employed to promote luminescence of cadmium sulfide (CdS) in an aqueous medium at room temperature.29 In this work, EA was exploited as an efficient electron donor for scavenging photogenerated holes on CdTe QDs and promoting the fluorescence intensity of CdTe QDs. Also, ethanol was added during the synthesis to improve the optical properties.
2. Experimental section
2.1 Materials
Cadmium chloride (CdCl2·2.5H2O, ≥99%) and tellurium powder (≥99.99%) were purchased from Sinopharm Chemical Reagent Co., Ltd. 3-Mercaptopropionic acid (MPA, 98%) and EA (99%) were purchased from the Aladdin Chemistry Co., Ltd. Sodium hydroxide (NaOH, ≥96%) was purchased from the Beijing Chemical Works. Sodium sulfide (Na2S·9H2O, ≥98%) was purchased from the Xilong Chemical Co., Ltd. Sodium borohydride (NaBH4, 96%) was purchased from Shanghai Chunsheng Fine Chemical Technology Co., Ltd. All chemicals were of analytical grade and used as received.2.2 Synthesis of aqueous CdTe QDs in the presence of erythorbic acid
Sodium hydrogen telluride (NaHTe) was prepared using a modified form of a method previously reported in the literature.30 Briefly, 0.4 mmol of tellurium powder and 2 mmol of NaBH4 were placed in a gas-tight syringe into which 10 mL of ultrapure water was drawn. Then the syringe was placed into a water bath at 80 °C. The metal pinhead of the syringe can be used to discharge the hydrogen that is produced. The syringe pinhead was joined to a pipe which was sealed from outside liquid. After 30 minutes, the black tellurium powder disappeared, and the pink NaHTe solution was prepared.Typically, the Cd source was prepared by adding 5 mL of 40 mM CdCl2 and 0.4 mmol of MPA into a beaker in sequence followed by adjustment of the pH to 8 with 5 M NaOH and then adding 10 mL of 20 mM EA. The pH was again adjusted to 8, and then the volume was adjusted to 30 mL with water. A portion of NaHTe (1 mL) was injected into the Cd source from the syringe. The reaction was carried out at 95 °C for three hours to synthesize the CdTe QDs. Samples of the QD solution obtained were taken and diluted with water for ultraviolet/visible (UV-Vis) spectroscopy and photoluminescence (PL) characterization. The samples were not purified before the characterization.
Then the as-prepared CdTe QDs were precipitated with excess ethanol, and the precipitate was separated by centrifugation and re-dissolved in water. This process was repeated several times to purify the CdTe QDs before X-ray diffraction (XRD) and high-resolution transmission electron microscopy (HRTEM) characterization.
2.3 Characterizations
UV-Vis absorption spectra were obtained using a Lambda 950 UV-Vis-near infrared (NIR) spectrophotometer. Fluorescence spectroscopy was performed with a Varian Cary Eclipse fluorescence spectrophotometer. XRD patterns were recorded with a Shimadzu XRD-6000 diffractometer. HRTEM analysis was conducted on a Jeol JEM 3010 microscope.3. Results and discussion
3.1 XRD and HRTEM analyses of the as-prepared CdTe QDs
Fig. 1 shows the XRD pattern of the as-prepared CdTe QDs. The peaks at approximately 2θ = 24.51°, 40.83° and 46.82° agree with those of zinc blende in the Joint Committee on Powder Diffraction Standards (JCPDS) database, indicating that the as-prepared CdTe QDs have a zinc blende structure. The HRTEM image of the as-prepared CdTe QDs (Fig. 2) shows that the CdTe QDs are well-dispersed spherical particles with a narrow size distribution and an average size of 3 nm. The quantum yield of the as-prepared CdTe QDs is 35%, which was measured according to the method described in the literature.313.2 Effect of the pH value of the Cd precursor
The pH value is an essential factor that strongly influences the optical performance of QDs prepared via aqueous synthesis.32 With a molar ratio of Cd/MPA of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, reaction temperature at 95 °C and reaction time of 3 h, the effect of pH values of the Cd precursor at 6.0–13.0 on the UV-Vis absorption spectra of the QDs was studied. It can be seen from Fig. 3, that with a decrease of pH, the absorbance of the solution slightly increased because of the large aggregates of CdTe. As the aggregates grew in size and the QDs continued to form a colloidal suspension, scattering increased. The absorbance measurements, which are sensitive to scattering from colloidal aggregates, are reflective of the dispersion of QDs in solution. A lower pH promotes the formation of free thiols and uncoated QDs. As a consequence, lowering the pH value enhances the formation of aggregates of as-prepared CdTe QDs.33 With a higher pH, the absorption spectra of the QDs became broader and showed a marked red shift. This phenomenon was because of the effect of the pH values on the surface S–H bond strength. With the increase of pH value, the binding force and the amount of capped Cd2+ increased, resulting in the formation of larger QDs. However, when the pH value was too high, the formation of Cd(OH)2 interfered with the QD surface, leading to a decline of optical properties. Therefore, a pH value of 8 was determined to be suitable for the experiments.
2, reaction temperature at 95 °C and reaction time of 3 h, the effect of pH values of the Cd precursor at 6.0–13.0 on the UV-Vis absorption spectra of the QDs was studied. It can be seen from Fig. 3, that with a decrease of pH, the absorbance of the solution slightly increased because of the large aggregates of CdTe. As the aggregates grew in size and the QDs continued to form a colloidal suspension, scattering increased. The absorbance measurements, which are sensitive to scattering from colloidal aggregates, are reflective of the dispersion of QDs in solution. A lower pH promotes the formation of free thiols and uncoated QDs. As a consequence, lowering the pH value enhances the formation of aggregates of as-prepared CdTe QDs.33 With a higher pH, the absorption spectra of the QDs became broader and showed a marked red shift. This phenomenon was because of the effect of the pH values on the surface S–H bond strength. With the increase of pH value, the binding force and the amount of capped Cd2+ increased, resulting in the formation of larger QDs. However, when the pH value was too high, the formation of Cd(OH)2 interfered with the QD surface, leading to a decline of optical properties. Therefore, a pH value of 8 was determined to be suitable for the experiments.
3.3 Effect of reaction temperature
The increase of reaction temperature from 25 °C to 200 °C had a significant effect on the UV-Vis absorption spectra of the as-prepared CdTe QDs as shown in Fig. 4. The reaction temperature, which controls the nucleation rate, is an important factor for the quality of the QDs.34 With a moderate increase in temperature from 25 °C to 100 °C, the QDs started to grow quickly, indicating that the growth of the QD crystals requires a relatively high temperature.12 The rising temperature affected the association of the CdTe complex, leading to the increase of the free monomer concentration in the subsequent growth. In the Ostwald ripening stage, it was expected that the equilibrium process existed between the stabilizer and the water molecules on the CdTe surface sites. However, when the temperature was too high, the rate of the dissociation of the ligands from the QD surface was accelerated,35 leading to a growth of crystals of CdTe QDs that was too fast to allow sufficient reaction with stabilizers.13 At temperatures greater than 100 °C, it was possible that fewer ligands occupied the surface sites, and more surface defects easily appeared because of the lower coverage provided by the ligands, suggesting that there existed a certain quantity of dangling bonds at the surface of the QDs.36 Thus, the optical properties of the CdTe QDs worsened. The experiments showed that the CdTe QDs prepared at 100 °C had the best optical properties.3.4 Effect of reaction time
Reaction time is another important factor for the synthesis of high-quality QDs. Fig. 5 shows the fluorescence spectra of the QDs prepared at 95 °C and different reaction times. The PL spectra of the as-prepared CdTe QDs shifted to longer wavelengths with the growth of CdTe QDs. With the increase of reaction time from 10 min to 30 min, the fluorescence intensity decreased slightly, and this was most likely because of the oxidation of the stabilizer molecules, which resulted in reduced stabilization of the ligands and increased the surface defects of the QDs.12 The fluorescence intensity increased when the reaction time was prolonged from 30 min to 6 h, and was accompanied with an obvious red shift of the maximum emission wavelength from 520 nm to 610 nm, reaching the maximum intensity with a reaction time of 6 h. The red shift of the position of the emission maximum and the spectral broadening with increasing reaction time were probably because of the increase in size and/or aggregation of the QDs.373.5 Effect of molar ratio of MPA/Cd
The proper passivation of the QD surface with ligands can produce high-quality semiconductor nanocrystals.38 MPA molecules on the surface sites instead of Te atoms favor the removal of the dangling bonds of Te atoms from the surface, and also prevent the oxidation of Te atoms.39 Abundant MPA coverage may eliminate the surface defects of the QDs that are generally adverse to luminescent emission. The molar ratio of MPA/Cd was varied from 1.5 to 16 to investigate the effect of stabilizer concentration on the optical properties of CdTe QDs. As shown in Fig. 6, with increasing MPA/Cd molar ratio, the UV absorption peak of the QDs became wider, which indicates a worsening of size focusing and a decrease in monodispersity. One possible explanation for these changes is that many ligand molecules may crosslink with each other on the surface of QDs, resulting in more surface defects. When the MPA/Cd ratio was 16, the UV absorption peak was red shifted. This red shifting can be explained by an excess amount of MPA in solution having led to an increased hydrolysis of MPA, and as a result, a higher sulfide S2− content in the reaction medium that accelerated the growth of the QDs.36 The excess monomers in the precursor solution can be consumed to cause a fast growth of CdTe QDs, leading to the coarse surface and poor optical properties. | ||
| Fig. 6 UV-Vis absorption spectra of the as-prepared CdTe QDs prepared with different ratios of MPA/Cd. | ||
3.6 Effect of EA dosage
The effects of EA dosage on the optical properties of CdTe QDs were investigated. It can be seen from Fig. 7a that with the increase of EA dosage, the absorption peak became blue shifted because of competing light-induced surface oxidation processes. As can be seen from Fig. 7b, there was an enhancement in the luminescence intensity when EA was included during QD synthesis. The intensity of the CdTe QD luminescence increased with increasing dosage of EA. This was because of the reduction of the oxidized states on the surface and the passivation of CdTe QDs by EA. It was reported that certain antioxidants consume the oxidized surface atoms resulting in the fluorescence restoration.40 Thus, it can be deduced that the formation of a Cd-erythorbate protective layer is responsible for the increase in the emission intensity. | ||
| Fig. 7 (a) UV-Vis absorption spectra of the as-prepared CdTe QDs prepared with different dosages of EA. (b) PL emission spectra of the as-prepared CdTe QDs prepared with different dosages of EA. | ||
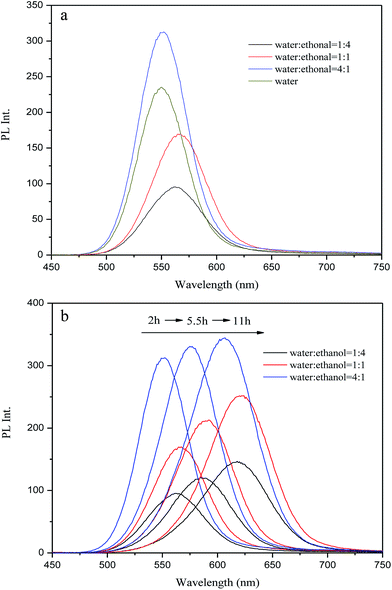
3.7 Effect of ethanol dosage
In comparison with other aqueous phase approaches, the main difference of the route in this work is the use of a mixture of ethanol and water as a solvent for the preparation of NaHTe. It can be seen from Fig. 8a that the fluorescence was enhanced with the increase of the water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol ratio from 1
ethanol ratio from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 to 4
4 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. When the water
1. When the water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol ratio was 4
ethanol ratio was 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the photoluminescence intensity was higher than that resulting from the approach with pure water as the solvent (i.e., “4
1, the photoluminescence intensity was higher than that resulting from the approach with pure water as the solvent (i.e., “4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0” ratio). The superior PL performance can be attributed to the particle aggregation that was caused by the weak hydrogen bonds of the stabilizer with ethanol.41 However, the PL intensity decreased as more ethanol was included during the synthesis. This decrease occurred because most MPA molecules, which were supposed to bond to the surface of the QDs, actually formed intermolecular hydrogen bonds with ethanol. Also, the quenching mechanism was attributed to the non-radiative recombination because of the esterification reaction that occurred between ethanol and the carboxylic group of the stabilizer.42 It can be seen from Fig. 8b that the first exciton peaks of the QDs are noticeably red shifted with the increase of the reaction time from 2 h to 11 h regardless of the water
0” ratio). The superior PL performance can be attributed to the particle aggregation that was caused by the weak hydrogen bonds of the stabilizer with ethanol.41 However, the PL intensity decreased as more ethanol was included during the synthesis. This decrease occurred because most MPA molecules, which were supposed to bond to the surface of the QDs, actually formed intermolecular hydrogen bonds with ethanol. Also, the quenching mechanism was attributed to the non-radiative recombination because of the esterification reaction that occurred between ethanol and the carboxylic group of the stabilizer.42 It can be seen from Fig. 8b that the first exciton peaks of the QDs are noticeably red shifted with the increase of the reaction time from 2 h to 11 h regardless of the water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol ratio.
ethanol ratio.
3.8 Effect of NaBH4 dosage
NaBH4 played two roles in the synthesis of CdTe QDs. The first was to lead to a fast reduction of Te to Te2− as shown in Scheme 1. The second was to supply a protective surrounds to avoid the oxidation of Te2− even without N2 protection. So an excess of NaBH4 was adopted. NaBH4 concentration had a significant effect on the conversion of Te to Te2−. In this work, NaBH4 was used as a reducing agent to treat the surfaces of thiol-capped CdTe QDs because NaBH4 was found to change the surface properties as well as the PL efficiency of some QD compounds of group II–VI elements.43 It can be seen from Fig. 9 that when the NaBH4 dosage was 1 mmol, which was a slight stoichiometric excess, the PL intensity was weak. This weakened intensity may be ascribed to NaBH4 oxidation. The PL intensity of the QDs increased markedly with the increase of NaBH4 dosage from 1 mmol to 2 mmol. However, the PL intensity decreased when the NaBH4 dosage further increased from 2 mmol to 10 mmol. Too much NaBH4 caused the detachment of thioglycolic ligands from the QD core, resulting in the aggregation and precipitation of QDs,43,44 thus worsening its optical properties. The PL spectra of the CdTe QDs prepared with different NaBH4 dosages exhibited emission peaks at the same wavelength with a narrow full width at half maximum (FWHM).4. Conclusions
This paper demonstrates a simple route to enhance the optical properties of QDs via the addition of EA and ethanol. Various parameters such as pH, reaction temperature, reaction time, and the amounts of and ratio of the reagents were investigated to optimize the synthesis, and luminescent CdTe QDs with different emission wavelength were synthesized. The key to this synthesis was the reducibility of EA, which provided a protective surrounding to avoid the oxidation of the QDs and MPA. The Cd-erythorbate protective layer led to CdTe QDs passivation, which was responsible for the enhancement of the optical properties. Ethanol was also found to improve the optical properties of the CdTe QDs because of its ability to form weak hydrogen bonds. The combination of EA and ethanol offers an easy and environmentally benign pathway for producing high quality photoluminescent QDs.Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 21176013), the Program for New Century Excellent Talents in University of China (NCET-12-0760), and the Fundamental Research Funds for the Central Universities (no. ZY1307).References
- Y. Shirasaki, G. J. Supran, M. G. Bawendi and V. Bulović, Nat. Photonics, 2013, 7, 13–23 CrossRef CAS.
- E. H. Sargent, Nat. Photonics, 2012, 6, 133–135 CrossRef CAS.
- K. I. Joo, Y. Fang, Y. Liu, L. Xiao, Z. Gu, A. Tai, C. L. Lee, Y. Tang and P. Wang, ACS Nano, 2011, 5, 3523–3535 CrossRef CAS PubMed.
- W. C. Law, R. Hu, C. K. Chen, G. Xu, X. Wang, I. Roy and K. T. Yong, RSC Adv., 2013, 3, 11511–11514 RSC.
- P. Singh, K. Joshi, D. Guin and A. A. Prabhune, RSC Adv., 2013, 3, 22319–22325 RSC.
- R. Gui, A. Wan, X. Liu, W. Yuan and H. Jin, Nanoscale, 2014, 6, 5467–5473 RSC.
- A. M. Smith and S. Nie, J. Am. Chem. Soc., 2010, 133, 24–26 CrossRef PubMed.
- J. Zhu, Y. Lu, Y. Li, J. Jiang, L. Cheng, Z. Liu, L. Guo, Y. Pan and H. Gu, Nanoscale, 2014, 6, 199–202 RSC.
- J. Li, C. Wu, P. Xu, L. Shi, B. Chen, M. Selke, H. Jiang and X. Wang, RSC Adv., 2013, 3, 6518–6525 RSC.
- C. Bhattacharya, Z. Yu, M. J. Rishel and S. M. Hecht, Biochemisty, 2014, 53, 3264–3266 CrossRef CAS PubMed.
- Z. Yu, R. M. Schmaltz, T. C. Bozeman, R. Paul, M. J. Rishel, K. S. Tsosie and S. M. Hecht, J. Am. Chem. Soc., 2013, 135, 2883–2886 CrossRef CAS PubMed.
- Y. Yu, L. Xu, J. Chen, H. Gao, S. Wang, J. Fang and S. Xu, Colloids Surf., B, 2012, 95, 247–253 CrossRef CAS PubMed.
- D. Zhou and H. Zhang, Small, 2013, 9, 3195–3197 CAS.
- D. Li, X. Liu, G. Xie and X. Liu, Colloids Surf., A, 2013, 424, 33–39 CrossRef CAS PubMed.
- T. Kabashima, Z. Yu, C. Tang, Y. Nakagawa, K. Okumura, T. Shibata, J. Lu and M. Kai, Peptides, 2008, 29, 356–363 CrossRef CAS PubMed.
- Z. Yu, T. Kabashima, C. Tang, T. Shibata, K. Kitazato, N. Kobayashi, M. K. Lee and M. Kai, Anal. Biochem., 2010, 397, 197–201 CrossRef CAS PubMed.
- J. A. Lines, Z. Yu, L. M. Dedkova and S. Chen, Biochem. Biophys. Res. Commun., 2014, 443, 308–312 CrossRef CAS PubMed.
- W. Yuan, H. Zhao, H. Hu, S. Wang and G. L. Baker, ACS Appl. Mater. Interfaces, 2013, 5, 4155–4161 CAS.
- C. Zhang, Y. Zu, X. Ji and Z. He, RSC Adv., 2014, 4, 20044–20047 RSC.
- Z. L. Zhu, L. Cui, T. Ling, S. Z. Qiao and X. W. Du, J. Mater. Chem. A, 2014, 2, 957–961 CAS.
- A. Hassinen, R. Gomes, K. De Nolf, Q. Zhao, A. Vantomme, J. C. Martins and Z. Hens, J. Phys. Chem. C, 2013, 117, 13936–13943 CAS.
- Y. Wang, R. Hu, G. Lin, W. C. Law and K. T. Yong, RSC Adv., 2013, 3, 8899–8908 RSC.
- S. W. Tong, N. Mishra, C. L. Su, V. Nalla, W. Wu, W. Ji, J. Zhang, Y. Chan and K. P. Loh, Adv. Funct. Mater., 2014, 24, 1904–1910 CrossRef CAS.
- X. Cao, F. Shen, M. Zhang, J. Bie, X. Liu, Y. Luo, J. Guo and C. Sun, RSC Adv., 2014, 4, 16597–16606 RSC.
- R. Hodlur and M. Rabinal, Chem. Eng. J., 2014, 244, 82–88 CrossRef CAS PubMed.
- D. Zhou, M. Lin, X. Liu, J. Li, Z. Chen, D. Yao, H. Sun, H. Zhang and B. Yang, ACS Nano, 2013, 7, 2273–2283 CrossRef CAS PubMed.
- X. Luo, J. Han, Y. Ning, Z. Lin, H. Zhang and B. Yang, J. Mater. Chem., 2011, 21, 6569–6575 RSC.
- D. Zhou, M. Lin, Z. Chen, H. Sun, H. Zhang, H. Sun and B. Yang, Chem. Mater., 2011, 23, 4857–4862 CrossRef CAS.
- Y. Liang, K. Yu, J. Wang, J. Chen, B. Sun and L. Shao, Colloids Surf., A, 2014, 455, 129–135 CrossRef CAS PubMed.
- L. Zou, Z. Gu, N. Zhang, Y. Zhang, Z. Fang, W. Zhu and X. Zhong, J. Mater. Chem., 2008, 18, 2807–2815 RSC.
- G. A. Crosby and J. N. Demas, J. Phys. Chem. C, 1971, 75, 991–1024 CrossRef CAS.
- Y. Nakane, Y. Tsukasaki, T. Sakata, H. Yasuda and T. Jin, Chem. Commun., 2013, 49, 7584–7586 RSC.
- A. Mandal and N. Tamai, J. Phys. Chem. C, 2008, 112, 8244–8250 CAS.
- H. Saito, K. Nishi and S. Sugou, Appl. Phys. Lett., 1999, 74, 1224–1226 CrossRef CAS PubMed.
- S. F. Wuister, F. van Driel and A. Meijerink, Phys. Chem. Chem. Phys., 2003, 5, 1253–1258 RSC.
- J. Guo, W. Yang and C. Wang, J. Phys. Chem. B, 2005, 109, 17467–17473 CrossRef CAS PubMed.
- D. Mutavdžić, J. Xu, G. Thakur, R. Triulzi, S. Kasas, M. Jeremić, R. Leblanc and K. Radotić, Analyst, 2011, 136, 2391–2396 RSC.
- J. Li, T. Yang, W. Chan, M. M. Choi and D. Zhao, J. Phys. Chem. C, 2013, 117, 19175–19181 CAS.
- W. Cai, L. Jiang, D. Yi, H. Sun, H. Wei, H. Zhang, H. Sun and B. Yang, Langmuir, 2013, 29, 4119–4127 CrossRef CAS PubMed.
- Y. J. Chen and X. P. Yan, Small, 2009, 5, 2012–2018 CrossRef CAS PubMed.
- W. G. Tian, W. Mi, J. T. Tian, J. Q. Jia, X. Y. Liu, Z. B. Zhu, J. H. Dai and X. Wang, Analyst, 2013, 138, 1570–1580 RSC.
- J. Abolhasani, E. Ghorbani-Kalhor, J. Hassanzadeh and S. B. Hoseini, J. Environ. Treat. Tech., 2013, 1, 213–216 Search PubMed.
- E. Jang, S. Jun, Y. Chung and L. Pu, J. Phys. Chem. B, 2004, 108, 4597–4600 CrossRef CAS.
- J. Ma, J. Y. Chen, J. Guo, C. C. Wang, W. L. Yang, N. H. Cheung and P. N. Wang, Nanotechnology, 2006, 17, 5875 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |