Recent advances in the synthesis of 2-substituted benzothiazoles: a review
Neelam P. Prajapati
,
Rajesh H. Vekariya
,
Mayuri A. Borad
and
Hitesh D. Patel
*
Department of Chemistry, School of Sciences, Gujarat University, Ahmedabad, India. E-mail: drhiteshpatel1@gmail.com; Fax: +91-079-26308545; Tel: +91-079-26300969
First published on 29th October 2014
Abstract
Benzothiazole can serve as unique and versatile scaffolds, especially in synthetic and pharmaceutical chemistry because of their potent and significant pharmacological activities. This important class of derivatives possess numerous pharmacological activities like antitumor, antimicrobial, anti-inflammatory, anticonvulsant, antidiabetic activities and so on. Many scientists have developed a wide range of methodologies for the synthesis of the 2-substituted benzothiazole nucleus and its derivatives using different types of catalysts to improve the selectivity, purity and yield of the products. Thus, the present review article focuses mainly on the different kind of reactions involved in synthesis of the 2-substituted benzothiazole nucleus and its derivatives.
1. Introduction
Benzothiazole is a privileged bicyclic ring system. It contains a benzene ring fused to a thiazole ring. The benzothiazole ring system is present in various marine and terrestrial natural compounds, which have a wide range of pharmaceutical applications.1–3 2-Aminobenzothiazoles are highly reactive compounds and extensively utilized as reactants or reaction intermediates for the synthesis of a variety of fused heterocyclic compounds.4 Medicinal chemists’ attention was drawn to this series when the pharmacological profile of riluzole (6-trifluoromethoxy-2-benzothiazolamine) (Fig. 1) was observed as a clinically available anticonvulsant drug.5 Furthermore, Erythrazoles A and Erythrazoles B were isolated from mangrove sediments (Fig. 1).6The benzothiazole moiety plays an important role in chemistry and is also present in a variety of biologically active applications such as anti-microbial,7–10 anti-cancer,11–16 anthelmintic,17 anti-diabetic,18,19 anti-tuberculotic,13,20,21 anti-tumor,22–28 anti-trypanosomal,29 anti-viral,30–32 antibacterial,33–36 anti-oxidant,37 anti-glutamate and anti-parkinsonism,38,39 analgesic,40 anti-inflammatory,41–43 antifungal,44,45 antileishmanial,46 anticonvulsant,47 neuroprotective,48 muscle relaxant activities,49 vasodilator,50 orexin receptor,51 inhibitors of several enzymes,52,53 atherosclerosis,54 insomnia,51 epilepsy,55 LTD4 receptor antagonists,56,57 antiparasitics and photosensitizers,58 calcium channel antagonists,59,60 as imaging agents for Aβ plaques in cerebral amyloid angiopathy,61 and as falcipain inhibitors.62 In addition they shows other applications such as radioactive β-amyloid imaging agents,63–67 and schistosomicidal agents.68 Due to its potent and significant biological activities it has great pharmaceutical importance; hence, synthesis of this compound is of considerable interest. The studies of structure–activity relationship (SAR) interestingly reveal that the change in the structure of the substituent group at C-2 position commonly results the change of its bioactivity.
A simple and efficient transformation using readily available reagents under solvent-free and metal-free conditions is considered a key solution for pollution problems generated by large-scale reactions. Thus, recently many protocols were developed for the synthesis of benzothiazole derivatives catalyzed by heterogeneous solid acid catalysts and ionic liquids, reactions under microwave irradiation as well as reactions performed under mild and solvent free conditions, which are included in this review. In addition, ultrasound promoted synthesis of benzothiazoles are also reported. The present article is intended to briefly review recent research progress concerning the synthesis of various benzothiazole derivatives via different methodologies, which mainly includes condensation of ortho-amino thiophenols and acids/acid chlorides/aldehydes/esters/nitriles/ketones/thioesters. In addition, ortho-amino thiophenols condensed with carbon disulfide (CS2)/sodium sulphide (Na2S) to afford various benzothiazole derivatives. Also, benzothiazole derivatives were synthesised through the condensation reaction of various ortho-amino thiophenols/ortho-halo amines/thiophenols with various isothiocyanates. Moreover, 2-amino benzothiazoles were synthesised using bromine (Br2) through the condensation reaction of various aromatic amines with ammonium thiocyanate (NH4SCN)/sodium thiocyanate (NaSCN)/potassium thiocyanate (KSCN). Benzothiazole derivatives were also synthesised by cyclization of different aromatic thiourea linkages. Moreover, the reaction of aromatic thiourea with Lawesson’s reagent also afforded benzothiazole derivatives.
2. Synthesis of benzothiazole
2.1 By condensation reaction
2.1.1.1 Homogeneous catalysis.
Acid catalyzed condensation. Acid catalyzed homogeneous condensation of ortho-aminothiophenol and substituted aldehyde using H2O2/HCl in ethanol at room temperature has been demonstrated by Guo and co-authors (Scheme 1).69
A series of novel 2-phenylbenzothiazoles was synthesized by Mortimer and colleagues by the reaction of ortho-aminothiophenol disulfides and substituted benzaldehydes under reducing conditions in ethanol (EtOH) (Scheme 2).70
(5-Benzothiazol-2-yl-furan-2-yl)methanol was obtained in quantitative yield from the condensation of hydroxymethylfurfural (HMF) and ortho-aminobenzenethiol in the presence of acetic acid (AcOH) was pointed out by Sattler and co-authors (Scheme 3).71
The para-phenylenediamine-2,5-di-(thiosulfuric acid) was heated with benzaldehydes and formed a benzal derivative as an intermediate, which at higher temperature yielded the benzobisthiazole reported by Perkin et al. (Scheme 4).72
Base catalyzed condensation. Maleki et al. have developed a mild and efficient protocol for the synthesis of 2-arylbenzothiazole derivatives from condensation of ortho-aminothiophenol with aromatic aldehydes employing ammonium chloride as a catalyst in methanol–water (15
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) as a dual solvent system at room temperature, which afforded high yields of the products (Scheme 5a and b).73 Here, the mechanism shows that ammonium chloride may first activate the carbonyl compounds by hydrogen bonding promoted the reaction via the nucleophilic attack of amines to afford the target molecules. The use of NH4Cl as a very inexpensive, metal-free and readily available reagent is the superior feature of this protocol. For comparative study, the authors have employed various organic solvents such as ethanol (EtOH), water (H2O), acetonitrile (ACN), dichloromethane (DCM) and chloroform (CHCl3). However, methanol–water has been proven the best solvent system in terms of yield. Short reaction time, high yield of the product, recycling of the catalyst, mild reaction conditions, acid-free and metal-free systems are the additional advantages of this method.
1 v/v) as a dual solvent system at room temperature, which afforded high yields of the products (Scheme 5a and b).73 Here, the mechanism shows that ammonium chloride may first activate the carbonyl compounds by hydrogen bonding promoted the reaction via the nucleophilic attack of amines to afford the target molecules. The use of NH4Cl as a very inexpensive, metal-free and readily available reagent is the superior feature of this protocol. For comparative study, the authors have employed various organic solvents such as ethanol (EtOH), water (H2O), acetonitrile (ACN), dichloromethane (DCM) and chloroform (CHCl3). However, methanol–water has been proven the best solvent system in terms of yield. Short reaction time, high yield of the product, recycling of the catalyst, mild reaction conditions, acid-free and metal-free systems are the additional advantages of this method.
Solvent catalyzed condensation. Bithienyl-1,3-benzothiazoles, another new derivative of benzothiazole, were prepared using the condensation reaction of ortho-aminobenzenethiol and various 5-formyl-5′-alkoxy- or 5-formyl-5-N,N-dialkylamino-2,2′-bithiophenes under refluxing dimethyl sulfoxide (DMSO) for 30–60 min by Batista et al. (Scheme 6).74 Assessment of the fluorescence properties of new benzothiazoles demonstrate that they show strong fluorescence between 450–600 nm.
Microwave-induced condensation. Praveen and co-workers disclosed the microwave-assisted one-pot synthesis of benzothiazole and benzoxazole derivatives using phenyliodoniumbis(trifluoroacetate) (PIFA) as an effective oxidant for the cyclocondensation of ortho-aminothiophenols/ortho-aminophenols with different aldehydes in ethanol at 80 °C, which afforded high yields of the products (Scheme 7).75 Heterocyclic nucleus containing substituted aldehyde compounds such as pyridine, thiophene and furan allowed an average yield due to the cleavage of these heterocycles under microwave irradiation. According to the possible reaction mechanism, an imine intermediate is formed. This protocol has advantages like PIFA (which works both as a Lewis acid and as an oxidant), wide substrate scope, short reaction time, microwave condition and satisfactory yields.
The condensation of appropriate 2-phenyl-1H-indole-3-carboxaldehyde and 5-substituted ortho-aminothiophenols in the presence of piperidine or para-toluenesulphonic acid (p-TSA) in ethanol (EtOH) or N,N-dimethylformamide (DMF) solvent under microwave irradiation (MWI) for 3–6 min at 240 W was reported by Dandia et al. (Scheme 8).76 Considerable increase in reaction rate and improved yields were observed in case of microwave irradiation compared to classical methods.
Paul et al. have developed a simple and efficient procedure for the synthesis of 2-arylbenzothiazoles by a one-pot condensation reaction of ortho-aminothiophenol with β-chlorocinnamaldehydes under microwave irradiation (MWI) using para-toluenesulfonicacid (p-TSA) (Scheme 9).77 Operational simplicity, fast reaction, environmentally friendly, general applicability and the ability to accommodate a variety of substitution patterns are the notable advantages of this procedure.
2.1.1.2 Heterogeneous catalysis.
Acid catalyzed condensation. Phosphorous pentoxide is an inexpensive reagent which acts as an acid catalyst. An efficient protocol for the synthesis of 2-arylbenzothiazole via condensation of various aldehydes and ortho-aminothiophenol in the presence of Phosphorus pentoxide (P2O5) in methanol (MeOH) for 3–7 h at room temperature was reported by Nalage et al. (Scheme 10, Method 1).78
The use of cobalt nitrate (Co(NO3)2·6H2O)/hydrogen peroxide (H2O2) as a novel and efficient reagent for the synthesis of 2-arylsubstituted benzothiazoles was investigated by Chandrachood and colleagues (Scheme 10, Method 2).79 They have noted the effect of changing the solvent, temperature and reagent quantity on the reaction and found the best results using cobalt nitrate and hydrogen peroxide in N,N-dimethylformamide (DMF). Aliphatic aldehydes do not give notable yield with this reagent.
Rapid and effective condensation reactions of ortho-aminothiophenol with various aldehydes were carried out using I2 in solvent-free conditions by Moghaddam et al. to afford the corresponding 2-substituted benzothiazole derivatives in a relatively short time in excellent yields (Scheme 10, Method 3).80
Weekes et al. have reported a simple, one-pot and high-yielding protocol for substituted 2-phenylbenzothiazoles under both thermal and microwave (MW) conditions from the condensation of various benzaldehydes and ortho-aminothiophenol using sodium metabisulfite (Na2S2O5) as a mild oxidant in dimethylsulfoxide (DMSO) at 120 °C (Scheme 10, Method 4).81 Here, a lower reaction time and the simple isolation of the product without column chromatography are the additional advantages of this method. Authors also achieved excellent yields of the product using dimethylformamide (DMF) at 90 °C as a solvent, though this reaction takes longer (>2 h) because of the lower solubility of reaction components in DMF. However, DMSO is an efficient solvent for promoting this reaction, due to its optimal reagent dissolution and oxidizing properties.
The synthesis of 2-(para-tolyl)benzothiazole by transition metal-Ir-catalyzed hydrogen-transfer reactions of 4-methylbenzaldehyde with ortho-aminothiophenol have been suggested by Blacker and colleagues (Scheme 10, Method 5).82
The H2O2/CAN system as a novel and very efficient reagent for the convenient synthesis of benzothiazoles in good to excellent yields through the condensation of ortho-aminothiophenol and various substituted aryl aldehydes was described by Bahrami and co-authors (Scheme 10, Method 6).83 A short reaction time, an easy and quick isolation method, excellent chemoselectivity and good yields are the main advantages of this procedure.
2-Substituted benzothiazole and benzoxazole were synthesized by the condensation of aldehydes with ortho-aminothiophenol or ortho-aminophenol respectively through a one-pot reaction by applying diethylbromophosphonate and tert-butylhypochlorite (t-BuOCl) in acetonitrile (MeCN) by Patil et al. (Scheme 11).84 Both the diethyl bromophosphonate and tert-butyl hypochlorite have been used for oxidative cyclization and intramolecular cyclization but the authors have extended this idea by oxidative cyclization of Schiff’s base prepared from the condensation of various aldehydes with ortho-aminophenol and ortho-aminothiophenol, which leads to the formation of benzoxazole and benzothiazole respectively. The reaction was carried out at different molar ratios of oxidant in various solvents and showed that the reactions proceeded well with 2 equiv. of both the oxidants in acetonitrile.
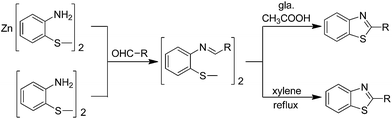
Bogert and co-authors have investigated a method for the synthesis of 2-substituted benzothiazoles by the condensation of zinc bis(ortho-aminothiophenolate) or ortho-aminophenyldisulphide and substituted aldehydes (Scheme 12).85 In the formation of thiazoles from the aminothiophenols and aldehydes, the authors have demonstrated that the thiazolines are intermediate products.
The condensation of ortho-aminothiophenol and 4-(diethylamino)-2-hydroxybenzaldehyde using PCl3 as a catalyst in ethanol (EtOH) was reported by Padalkar et al. (Scheme 13).86
Solid supported condensation. Maleki et al. have suggested an efficient and improved catalyst, sulphuric acid immobilised on silica-gel (H2SO4·SiO2) for the synthesis of 2-arylbenzothiazoles through the condensation of various aldehydes and ortho-aminothiophenol (Scheme 14, Method 1).87 Here, H2SO4·SiO2 is an inexpensive, heterogeneous and stable catalyst possessing very high reactivity compared to unsupported H2SO4. The authors have examined the effect on the yield by employing different amounts of catalyst in various solvents but considerable growth of the reaction rate and improvement of the yield were observed when 5 mg of H2SO4·SiO2 in ethanol (EtOH) was used.
Shokrolahi et al. have reported the condensation of ortho-aminothiophenol with aldehyde using Sulfonated Porous Carbon (SPC) as a heterogeneous catalyst in water under reflux conditions and microwave irradiation to produce benzothiazole derivatives (Scheme 14, Method 2).88 Here, porous carbon materials can accomplish most of the required properties for a suitable catalyst support due to high surface areas and well-developed porosities. Authors have studied the optimization using different amounts of SPC for the condensation reaction in water by refluxing (90 min) or by microwave irradiation (6 min). Use of 0.1 g of SPC shows the best results under these reaction conditions. The reusability and recyclability of the catalyst (SPC) was checked under similar reaction conditions and it was concluded that for the three catalytic cycles the yields and reaction times remained the same. The present protocol has a simple work up, is environmentally benign, good yields, no requirement of extra oxidants and the use of a cheap catalyst compared to previously reported methods.
The efficient synthesis of 2-substituted benzothiazoles in good yields by the reaction of ortho-aminothiophenol and various aldehydes in the presence of a catalytic amount of perchloric acid–doped polyaniline (HClO4–PANI) under refluxing ethanol (EtOH) was reported by Alibeik et al. (Scheme 14, Method 3).89 The superior characteristics of this catalyst are its low cost, simple recovery and efficient reusability. The authors have studied the reusability of the catalyst and the results show that there is no considerable change in catalyst reactivity. There is only a steady downfall in the time and yield of the reaction till the third time of reuse, but this moderate deactivation is stopped in the fourth run.
Alloum et al. have presented the condensation of various aldehydes with ortho-aminothiophenol on silica gel/nitrobenzene or montmorillonite K-10/nitrobenzene under microwave irradiation (MWI), affording 2-arylbenzothiazoles in good yields with high purity (Scheme 14, Method 4).90
Kawashita and co-workers have disclosed a simple and direct synthesis of 2-arylbenzothiazole with the aid of activated carbon (Shirasagi KL) (Scheme 14, Method 5).91 2-Pyridylbenzothiazole prepared by this method was proven to work as a ligand in a palladium-catalyzed Mizoroki–Heck reaction.
Kumar and co-authors have attempted a synthesis of a library of 2-substituted benzothiazoles via the condensation of various substituted amines with different aldehydes in the presence of poly[4-diacetoxyiodo] styrene (PDAIS) as a solid supported hypervalent iodine reagent in dichloromethane (DCM), which afforded an excellent yield of the products (Scheme 15).92 A general mechanism for PDAIS mediated synthesis of benzothiazoles showed that an imine intermediate is generated during the reaction of an amine with aldehyde, which undergoes intramolecular cyclization to afford the final product. In addition, PDAIS is converted to polymer supported iodobenzene which is recovered by filtration is the major benefit of this protocol.
Polymer supported condensation. Deligeorgiev and co-authors have surveyed a reliable, simple, highly reproducible and an improved green protocol for the preparation of 2-aryl- and 2-hetaryl-benzothiazoles by the condensation of equivalent amounts of 2,2-diaminodiphenyldisulfides (method A) or ortho-aminothiophenols (method B) and diverse aromatic aldehydes applying PEG 200/400 under microwave irradiation (MWI) (Scheme 16).93 Here, the short reaction time, easy work-up procedure, high purity of the products without the use of column chromatography, high yield and the use of green solvent PEG 200 or PEG 400 are the superior advantages of this protocol. Moreover, for the optimization of reaction conditions, the authors carried out this reaction in microwave irradiation. However, the best results were obtained by irradiation of the reaction mixture for 5 to 15 min at 560 W in the presence of para-toluenesulfonic acid (p-TSA) and green solvent PEG 200/400.
Resin supported condensation. Lee and co-workers investigated the solid phase synthesis of benzothiazole derivatives using Wang resin and Rink amide resin, which was filled by bis-(2-nitro-4-carboxyphenyl) disulfide. The nitro group reduced to its amine by rupture of the disulfide bond using SnCl2·2H2O to afford 1-(2-nitro-4-carboxyphenyl)thiol with Wang resin and Rink amide resin, which was condensed with α–β substituted aldehyde in the presence of ethanol under reflux condition, followed by trifluoroaceticacid (TFA) cleavage and spontaneous oxidation to afford benzothiazole derivatives (route A). The bisnucleophilic attack of thiol with Wang resin and Rink amide resin on an unsaturated ketone in the presence of 1% CH3COOH in ethanol under reflux condition gives 2,3-dihydro-[1,5]-benzothiazepine derivatives (route B) (Scheme 17).94
Sadjadi et al. have found a new solvent free approach for the synthesis of 2-arylbenzothiazole in the existence of MCM-41 supported Cu(OAc)2, as a catalyst under ultrasonic irradiation to afforded excellent yield of the products (Scheme 18).95
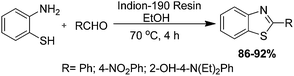
A mild protocol has been developed for the preparation of benzothiazoles from reactions of aldehydes with ortho-substituted amino aromatics in the presence of catalytic amounts of Indion 190 resin by Padalkar and co-authors (Scheme 19).96 In order to find the optimum reaction conditions, the reaction has been carried out between ortho-phenylenediamine and benzaldehyde in the presence of different catalysts and solvents at various temperatures, and the results clearly shows the effective use of Indion 190 resin and ethanol (EtOH) as a solvent for the preparation of 2-phenylbenzimidazole. Authors have also determined that lower temperatures require more time for the completion of the reaction and contrarily, a higher amount of catalyst increases the acidity of the reaction medium, so the yield of the corresponding product decreases.
Organo-silicon supported condensation. A mild and efficient reagent chlorotrimethylsilane (TMSCl) in dimethylformamide (DMF) as a promoter and water scavenger and Fe(NO3)3 as catalyst for the one-pot preparation of benzothiazoles from aromatic ortho-aminothiophenols and aldehydes under ultrasonic irradiation with good yield was explored by Yuan and colleagues (Scheme 20).97 The authors performed a set of experiments on 4-phenylsulfanyl-benzaldehyde and ortho-aminothiophenol by applying various amounts of TMSCl and Fe(NO3)3 at different reaction temperatures and deduced that the best result was achieved by carrying out the reaction with TMSCl and Fe(NO3)3 (4
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 60 °C under ultrasonic irradiation in DMF or without solvent.
1) at 60 °C under ultrasonic irradiation in DMF or without solvent.
Kodomari and colleagues have established a simple, convenient and rapid method for the synthesis of 2-substituted benzothiazoles by the condensation of aldehydes and ortho-aminothiophenol under microwave irradiation (MWI) in the presence of silica gel under solvent free conditions (Scheme 21).98 The silica gel could be easily recovered and reused for additional reactions without loss of activity. Here, solvent-free conditions, use of non-toxic catalysts, the high yield of the products and shorter reaction times are the advantages of this protocol.
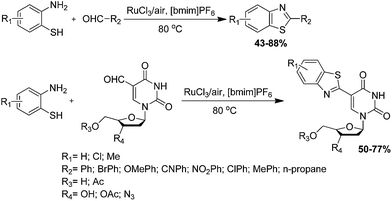
Ionic-liquid catalyzed condensation. Fan et al. have developed an efficient, environmentally beneficial and practical procedure for the synthesis of 2-substituted benzothiazoles via oxidative condensation of ortho-aminobenzenethiol with various aldehydes in the presence of RuCl3 as a catalyst in [bmim]PF6 ionic liquid as the reaction medium and air as the oxidant (Scheme 22a and b).99 The authors have also studied the reaction under different conditions with regard to various catalysts, solvent, temperature and reaction time. However the best results were obtained in the case of 80 °C in [bmim]PF6 as a reaction media.
Fan et al. have developed a method for the synthesis of 2-benzoylbenzothiazoles and 2-benzylbenzothiazoles via condensation of ortho-aminothiophenols and phenylacetaldehydes using FeCl3·6H2O as an oxidant and [bmim]BF4 ionic liquid as both reaction medium and co-catalyst, which afforded excellent yield of the products (Scheme 23).100
Using bio-reagent. The cyclocondensation of ortho-aminothiophenol and aldehydes to produce 2-arylbenzothiazole and 2-heteroaryl benzothiazoles was discovered by Pratap and co-workers in dichloromethane (DCM) using bakers’ yeast as a catalyst in good yields. This protocol could be an eco-friendly and attractive way for the synthesis of highly functionalized bioactive benzothiazoles. To evaluate the effect of the solvent, the authors have carried out the model reaction in different solvents such as water (H2O), ethanol–water, ethanol (EtOH), methanol (MeOH), 1,4-dioxane, acetonitrile (ACN), N,N-dimethylformamide (DMF) and dichloromethane (DCM). However, the yield of benzothiazoles was found to be best in dichloromethane (Scheme 24).101
Riadi et al. have efficiently synthesized a series of benzothiazoles by the condensation of ortho-aminothiophenol with aromatic aldehydes in the presence of catalytic amounts of Animal Bone Meal (ABM) and Lewis acids doped ABMs under reflux conditions in air (Scheme 25).102 The remarkable features of this new protocol are high conversion, short reaction times, cleaner reaction profiles, straight forward procedure and reduction in catalyst toxicity.
Metal nanoparticle catalysed condensation. An efficient procedure for the synthesis of 2-substituted benzothiazoles was employed using ortho-aminothiophenol and substituted aldehydes in the presence of Al2O3–Fe2O3 nanocrystals (5% w/w of ortho-aminothiophenol), as a heterogeneous catalyst at 60 °C by Bandyopadhyay and colleagues (Scheme 26).103
Zandt and colleagues have reported the synthesis of 4-fluoro-2-hydroxy-N(4,5,7,-trifluoro-benzothiazol-2-ylmethyl)-benzamide using N-cyanomethyl-4-fluoro-2-hydroxy-benzamide and 2-amino-4,5,7-trifluorothiophenol hydrochloride in refluxing ethanol (EtOH) for 24 h (Scheme 27, Method 2).105
Copper acetate catalysed the formation of 2-substituted benzothiazoles in excellent yields via the condensation of ortho-aminobenzenethiols with wide range of nitriles containing different functional groups was developed by Sun et al. (Scheme 27, Method 3).106 Optimization of the reaction conditions was explored and the optimal catalytic conditions consist of Cu(OAc)2 (10 mol%) and Et3N (1.0 equiv.) in ethanol (EtOH) at 70 °C for 6 h. According to the investigation of the reaction mechanism, sulfilimine formation and intramolecular cyclization occurs to furnish benzothiazoles.
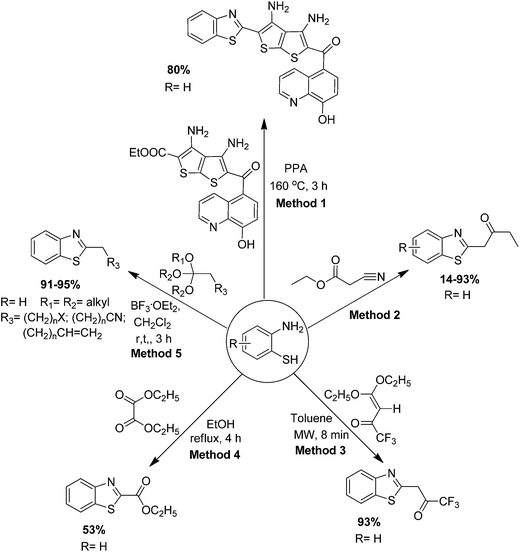
2.1.3.1 Condensation ortho-aminothiophenol with simple ester. Khalil et al. have determined that an amino ester and the selected ortho-substituted aromatic amines such as ortho-aminothiophenol was condensed in the presence of poly phosphoric acid (PPA) at 160 °C for 3 h followed by neutralization with aq. ammonia to yield the corresponding 2-substituted benzothiazole (Scheme 28, Method 1).107
Manfroni et al. have synthesized the 5-substituted ethyl-2-(benzothiazol-2-yl)acetate by the condensation of various substituted ortho-aminothiophenol and ethyl cyanoacetate at 120 °C, which afforded a high yield of the products (Scheme 28, Method 2).108
Reddy et al. have investigated the distinctive formation of trifluoroacetonyl benzothiazole by the condensation of ortho-aminophenol with trifluoroacetyl ketene diethyl acetal under microwave irradiation (MWI) in toluene for 8 min (Scheme 28, Method 3).109
Shantakumar et al. have presented some new benzothiazole derivatives by the reaction of benzothiazolyl carboxyhydrazide with variant aryl acids using phosphoryl chloride (POCl3) (Scheme 28, Method 4).49
The convenient, flexible, connective and efficient preparation of various benzothiazole derivatives by the condensation of substituted anilines with functionalized orthoesters in good to excellent yields under mild conditions was established by Bastug and colleagues (Scheme 28, Method 5).110 The development of new libraries of multifunctional sites containing heterocycles is the versatility of this protocol. A broad range of variety of substituted orthoesters were prepared using the modified Pinner sequences which widen the scope of this condensation.
2.1.3.2 Condensation ortho-aminothiophenol with thionoester. Karlsson and co-workers have described the protocol for the synthesis of (2,6′-bibenzothiazole)-2′-thiol via the condensation of 4-(benzothiazole-2-yl)-2-bromoaniline with potassium ortho-ethyl dithiocarbonate using dimethyl formamide (DMF) at 140 °C for 4 h (Scheme 29, Method 1).111
Yang et al. have suggested a synthetic approach for a highly efficient assembly of polyfluorinated 2-benzylthiobenzothiazoles by readily available starting materials such as various substituted anilines and potassium ortho-ethyl dithiocarbonate, which require shorter reaction times and allow good to excellent yield (Scheme 29, Method 2).112
Yildiz et al. have reported a viable methodology for the synthesis of various 2-substituted benzothiazoles from ortho-aminothiophenols and corresponding carboxylic acids refluxing in trimethylsilylpolyphosphate ester (PPSE) at various temperatures and time to afford various derivatives in excellent yield (Scheme 30, Method 2).114
Molecular iodine was employed by Gupta and co-workers in a one-pot, solid-phase, solvent-free and microwave assisted reaction of ortho-aminothiophenol and various benzoic acids to obtain high yields of various benzothiazole derivatives, compared to polyphosphoric acid (PPA) and [pmim]Br catalyzed microwave assisted reactions (Scheme 30, Method 3).115 The reaction was completed within 10 min and requires a very small amount of iodine. The authors have determined that this new protocol has a lower cost compared to PPA and [pmim]Br, because no additional chemicals and solvents are essential during this transformation. This protocol is an inexpensive, solvent-free and less time consuming.
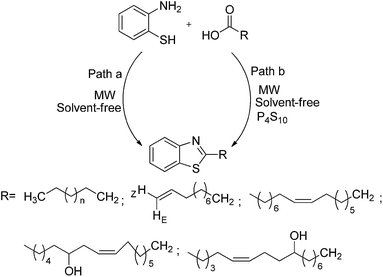
Rauf et al. have proposed a rapid, efficient and solvent-free one-pot synthesis of 2-substituted benzothiazole by the condensation of various fatty acids with ortho-aminothiophenol using P4S10 as a catalyst under microwave irradiation (MWI), which afforded a high yield of the products (Scheme 31).116 The reaction was completed within 3–4 min with good yields in the presence of a catalyst (path-b), while it takes 30 min without catalyst to obtain poor yields (path-a).
An efficient and one-pot synthesis of 2-trifluoro- and 2-difluoromethyl substituted benzothiazole derivatives in excellent yields by the condensation reaction of trifluoroacetic acid and difluoroacetic acid with commercially available ortho-aminobenzenethiols, respectively, was examined by Ge and co-workers (Scheme 32).117
2.1.5.1 Condensation of ortho-aminothiophenol with acyl chloride. Racane et al. have reported that the bis-nitrile and nitro-nitrile derivatives of 2-phenylbenzothiazole were prepared by the condensation reaction of cyano or nitro-substituted ortho-aminobenzothiole with commercially available 4-cyano or 4-nitrobenzoylchloride, respectively, under reflux conditions in acetic acid (AcOH) for 4 h (Scheme 33, Method 1).118
An efficient and environmentally friendly catalyst NaHSO4–SiO2 promoted solvent-free synthesis of a library of benzothiazole derivatives by the condensation reaction of various acyl chlorides with ortho-aminothiophenol was developed by Kumar and co-workers (Scheme 33, Method 2).119 This reaction is heterogeneous in nature, so the catalyst can be easily recovered by simple filtration. The use of nontoxic, inexpensive, easily available, reusable and green catalyst makes the reaction protocol inexpensive and eco-friendly. The NaHSO4–SiO2 catalyst can be used four times with consistent yield, which is important for large scale operations and from an industrial point of view. Authors have also investigated the effect of time, temperature and solvent on the reaction. From this study they have concluded that the best results were obtained when NaHSO4–SiO2 was refluxing at 100 °C for 12 h without solvent.
A novel one-pot regioselective synthesis of 2-aryl benzothiazoles has been developed by Nadaf and co-worker using 1-butylimidazolium tetraflouroborate ([Hbim]BF4) and 1,3-di-n-butylimidazoliumtetrafluoroborate ([bbim]BF4) ionic liquids (ILs) as reaction media at room temperature in excellent yields (Scheme 33, Method 3).120 Ambient reaction conditions, absence of a catalyst and recyclability of the non-volatile ILs makes this protocol green and environment-friendly.
Karlsson et al. have made a minor change in the condensation reaction of ortho-aminothiophenol with 4-nitrobenzoyl chloride by applying N-methyl-2-pyrrolidone (NMP) as an oxidant at 100 °C for 1 h to give 2-(4-nitrophenyl)benzothiazole (Scheme 33, Method 4).121
Novel dibenzothiazole derivatives were synthesized by Wu and colleagues, in which zinc salt of 4-amino-3-mercaptobenzoic acid was suspended in pyridine with para-nitro benzoyl chloride at 80 °C for an hour to produce 2-(4′-nitrophenyl)-6-(benzothiazolyl)benzothiazole, which was converted to 2-(4′-nitrophenyl)-benzothiazole-6-carbonyl chloride by treatment with thionyl chloride (SOCl2) (Scheme 34).122 To a suspension of 2-(4′-nitrophenyl)-benzothiazole-6-carbonyl chloride in chlorobenzene, 5-substituted aminothiophenols were added, heated to reflux for 3 h to afford di-benzthiazole containing compounds. Both benzothiazole ring were prepared using condensation of acid chloride with ortho-aminothiophenol.
2.1.5.2 Condensation of ortho-iodoaniline with acyl chloride. Ding et al. have found a metal-free, simple and efficient one-pot synthesis of 2-substituted benzothiazoles by the condensation reaction of ortho-iodoanilines and various acid chlorides in the presence of Lawesson’s reagent, which afforded good to excellent yields of the products (Scheme 35).123 The predicted reaction mechanism for this protocol was determined to be the ortho-iodoanilines reacted with acid chlorides to afford benzamides, which became benzothioamides in the presence of Lawesson’s reagent and intramolecular cyclization of benzothioamides generates the expected benzothiazoles. Easy accessibility of starting materials, high efficiency, good substrate generality and suitability to combinatorial format are the attracting features of this protocol.
2.1.6.1 Condensation of ortho-aminothiophenol with isothiocyanate. El-Sharief and colleagues have introduced an exclusive condensation reaction of 1,4-phenylenediisothiocyanate with ortho-aminothiophenol to produce N,N′-bis-(benzothiazole-2-yl)-benzene-1,4-diamine using triethanolamine/N,N-dimethylformamide (TEA/DMF) as a reaction media (Scheme 36).124
Ghorabet al. have reported the condensation reaction of ortho-aminothiophenol with isothiocyanate derivative of thiophene for the synthesis of 5-(benzothiazole-2-yl-amino)-3-methylthiophene-2,4-dicarboxylicacid diethyl ester in tetrahydrofuran (THF) under reflux conditions for 6 h, which afforded good yields of the product (Scheme 37).125
2.1.6.2 Condensation of ortho-iodoaniline with isothiocyanate. Cano et al. have described the similar reaction of equimolecular amounts of ortho-iodoaniline and various isothiocyanato derivatives in presence of combination of dimethyl sulfoxide (DMSO) and an excess of potassium hydroxide (KOH) at 120 °C for 96 h, which afforded N-substituted benzothiazol-2-amine derivatives with good yields (Scheme 38, Method 1).126 The predicted pathway of the reaction mechanism shows that the benzyne intermediate was formed in situ from the ortho-iodoaniline, which was act as a strong electrophile. The reaction could be performed with a broad range of substrates, which gives this protocol high synthetic utility.
A copper(I)-catalysed tandem reaction of ortho-iodobenzenamine with isothiocyanates under mild conditions for the synthesis of 2-substituted benzothiazoles in good yield was disclosed by Ding et al. (Scheme 38, Method 2).127 High efficiency, mild reaction conditions and experimental ease are the key features of this method. Authors have explored various ligands and bases in different solvents for the optimization of the reaction conditions. From that study they have concluded that the best results were obtained when the reaction of ortho-iodoaniline and phenyl isothiocyanates are catalysed by CuI(I) (10 mol%) in the presence of ligand (1,10-phenanthroline) and base (1,4-diazabicyclo[2.2.2]octane) (DABCO) in toluene at 50 °C.
Guo et al. have reported a ligand- and base-free copper-catalyzed reaction of ortho-halobenzenamine derivatives with various isothiocyanates using copper(I)bromide (CuBr) and tetra-n-butyl ammonium bromide (TBAB-additive) as the promoter at 40 °C, affording various 2-aminobenzothiazoles in moderate to excellent yields (Scheme 38, Method 3).128
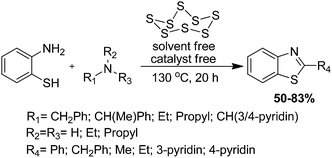
2.1.6.3 Condensation of substituted amine with isothiocyanate. Le and colleagues have investigated a one-pot methodology for the synthesis of various substituted 2-aminobenzothiazoles in high yield from phenyl isothiocyanate and various amines in the presence of 1-butyl-3-methylimidazoliumtribromide ([Bmim]Br3) and 1-butylmethylimidazoliumtetraflouoroborate ([Bmim]BF4) ionic liquids (Scheme 39).129 This method is highly useful because of its superior advantages like the recovery of the ionic-liquid many times without loss of its activity.
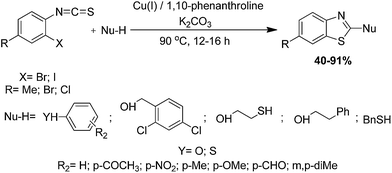
2.1.6.4 Condensation of substituted benzenethiol with isothiocyanate. Murru and co-authors have developed an efficient cascade method for the synthesis of a series of 2-substituted-1,3-benzothiazoles directly by intramolecular C–S bond formation from ortho-haloarylisothiocyanates and various O or S containing aromatic nucleophiles catalyzed by Cu(I)–L (1,10-phenanthroline) (Scheme 40).130 The thiocarbamate or dithiocarbamate generated in situ by the reaction of ortho-haloaryl isothiocyanates with O or S containing aromatic nucleophiles. This methodology is viable for the efficient synthesis of both O- and S-substituted 1,3-benzothiazoles with equal ease. On the other hand, alcohols and thiols were less reactive in this protocol.
The reaction of various derivatives of ortho-aminobenzenethiol with various ketones to yield 2,2-disubstituted benzothiazolines, which converted into 2-substituted benzothiazoles by pyrolysis with the elimination of concomitant hydrocarbon under reflux conditions have been examined by Elderfield and colleagues (Scheme 42, Method 2).133
Kreysa and co-workers have investigated a new protocol for the synthesis of 2-methylbenzothiazole using benzyl methyl ketone and ortho-aminobenzenethiol. During this reaction, toluene results as a by-product (Scheme 42, Method 3).134
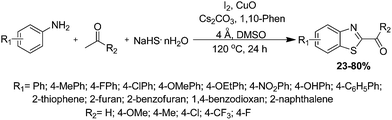
The condensation reaction of simple and readily available aromatic ketones with substituted anilines by employing NaSH·nH2O and CuO/CuI in the presence of base (Cs2CO3) and ligand (1,10-phenanthrolin) in dimethyl sulfoxide (DMSO) at 120 °C for the preparation of 2-acylbenzothiazoles, which assembles six reactions in one-pot was reported by Xue and co-authors (Scheme 43).135
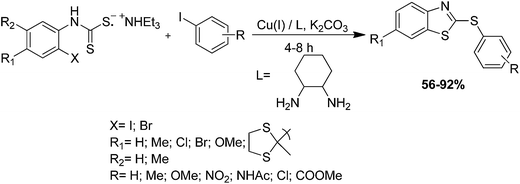
Shi and colleagues have surveyed a facile and diverse protocol for assembling both aliphatic and aromatic 2-thio-substituted benzothiazoles from ortho-iodoanilines and substituted aromatic or aliphatic thiol (RSH) using conveniently available reagents such as carbon disulfide (CS2), base (K2CO3) and catalyst (CuBr) which afforded good yields of the products (Scheme 44, Method 2).137 Condensation of carbondisulfide with thiols in the presence of K2CO3 generates carbonotrithioate salts in situ, which undergo coupling with ortho-iodoanilines and subsequent intramolecular condensation and elimination under assistance of CuBr to afford 2-thiosubstituted benzothiazoles.
Ma and colleagues have identified that the reaction of dithiocarbamate salts, which are excellent coupling partners for copper-catalyzed arylation, with substituted ortho-haloanilines can undergo intramolecular condensation in the presence of base (K2CO3) and dimethylformamide (DMF) to afford 2-N-substituted benzothiazoles in good yield (Scheme 44, Method 3a and b).138 This protocol enables the library of 2-N-substituted benzothiazoles with great diversity.
2-Methylbenzothiazole-5-carboxylicacid was obtained from 4-chloro-3-nitrobenzoicacid in a one-pot reaction using sodium sulfide (Na2S) in the presence of acetic anhydride (Ac2O) and acetic acid (HOAc) was reported by Klar and co-authors (Scheme 45).139
Inspired by the above developments, Xiang et al. has determined that a similar transformation would occur to synthesize 2-substituted benzothiazoles if the in situ generated carbanions acted as nucleophiles instead of N and/or S nucleophiles in this cascade three-component reaction in the presence of bases. Nitrogen-containing bisphosphonates (N-BPs), 2-methylbisphosphonate-substituted benzothiazoles, α-aryl-substituted nitriles, and α-benzothiazol-substituted nitriles could readily be obtained by employing bis(diethoxyphosphoryl)methanides and cyano(phenyl)methanides, generated in situ respectively, as nucleophiles with the optimized reaction conditions (1.2 equiv. CS2, 3 equiv. BuOK, 1 equiv. CuCl2 in DMF at 30 °C for 12 h) in hand (Scheme 46).140 Two series of extremely useful 2-C-substituted benzothiazoles containing gem-bisphosphonates and aryl-substituted nitriles were synthesized. The yields of products were severely affected by the substituent’s properties. It was also observed that weak electron-donating and electron-withdrawing substituents such as –Me and –F gave similar or slightly lower yields than 2-iodoaniline without any substituent. However, when stronger electron-donating and electron-withdrawing substituents such as –OMe and –COOMe were introduced into the benzene ring, the expected products were obtained in moderate yields. N-BPs are very useful in numerous pharmaceuticals and agrochemicals due to their broad range of biological activities and are also very valuable building blocks to gain amides, carboxylic acids, primary amines, ketones, heterocycles and biologically active compounds with or without the nitrile group.
Pan and colleagues have developed a simple, one-pot and an eco-friendly methodology for the synthesis of various substituted benzothiazoles by three-component reactions of ortho-iodoaniline, quaternary ammonium salt and sulfur powder in water with moderate to excellent yields up to 95% (Scheme 47).141 Quaternary ammonium salt works both as a phase transfer reagent and an alkylation reagent. Authors have also proposed a plausible reaction pathway which shows that the quaternary ammonium salt first reacted with sulfur powder to generate dialkyl disulphide, which would react with ortho-iodoaniline to give 2-(alkylthio)benzenamine. Then, the intramolecular cyclization reaction of 2-(alkylthio)benzenamine occurs to yield the targeted product.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of xylene and tetrahydrofuran (THF) by adding DMAD and then heating with the corresponding ortho-substituted aniline without the isolation of thioacylchloride.
1 mixture of xylene and tetrahydrofuran (THF) by adding DMAD and then heating with the corresponding ortho-substituted aniline without the isolation of thioacylchloride.
The formation of 2-aryl- and 2-alkyl-substituted benzothiazoles, benzimidazoles and benzoxazoles in excellent yield by polyphosphoricacid (PPA) catalyzed condensation of carboxylic acid, ester, amide or nitrile with an ortho-mercapto-, ortho-hydroxy- or ortho-amino-arylamine, respectively, has been examined by Hein and co-authors (Scheme 48, Method 2).143
Substituted benzothiazole derivatives were synthesized using condensation reactions of γ-lactone and 3-carboxy- or 3-cyano-4-methylcoumarin derivatives with ortho-aminothiophenol at 200 °C for 2 h using polyphosphoric acid (PPA) as a reagent was reported by Melikyan and co-workers (Scheme 48, Method 3a and b).144
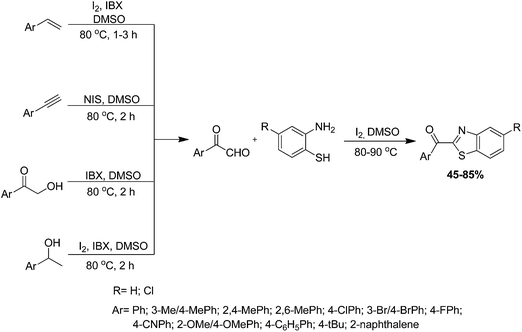
A variety of 2-acylbenzothiazoles were synthesized Zhu et al. using a multipathway coupled domino approach from the multiform substrates arylethenes, arylacetylenes, 2-hydroxy-aromatic ketones and 1-arylethanol via four distinct pathways. They converted into aryl substituted glyoxal in situ, which condensed with various ortho-aminothiophenol in a one-pot metal-free reaction (Scheme 49).145 This synthetic approach embodied four specific reaction pathways. For optimisation of reaction conditions, the authors have carried out this reaction in the presence of various oxidants and additives in dimethyl sulfoxide (DMSO). However, the excellent reaction conditions for this reaction turned out to be styrene (1.1 mmol) and ortho-aminobenzenethiol (1.2 mmol) using iodine/ortho-iodobenzoic acid (I2/IBX, 2.0 mmol/1.5 mmol) in DMSO at 80 °C. Arylethenes, 2-hydroxy-aromatic ketones and 1-arylethanol follow the same optimised procedure, but arylacetylenes occur in good yield using N-iodosuccinimide (NIS) as catalyst. In pursuance of the possible reaction mechanism, all four substrates are converted into phenacyl iodine through consecutive iodination and oxidation in I2/IBX or NIS, which is further converted to phenylglyoxal in DMSO. Finally, phenylglyoxal reacted with ortho-aminothiophenol via condensation to afford the desired product.
2.2 By cyclization reaction
A wide range of derivatives of benzothiazole were synthesized by the cyclization of various substituted thioformanilides using different reagents and novel methods.Rey and co-authors have investigated a simple and affordable methodology for the synthesis of 2-substituted benzothiazoles by the photochemical cyclization of thioformanilides propelled by chloranil under irradiation in 1,2-dichloroethane (DCE) and toluene at 80 °C (Scheme 50, Method 1).146 The key step of the reaction mechanism was hydrogen atom abstraction from thiobenzamide by triplet chloranil.
Another aerobic visible-light promoted photo redox catalytic formation of 2-substituted benzothiazoles has been accomplished by Cheng et al. via radical cyclization of thioanilides without metal involvement except the sensitizer (Scheme 50, Method 2).147 Various catalyst and solvents were applied for optimization of the reaction conditions and the results shows that tris(bipyridine)ruthenium(II)hexafluorophosphate (Ru(bpy)3(PF6)2) works as an optimal catalyst and N,N-dimethyl formamide (DMF) works as an optimal solvent for this new protocol. Visible-light as the reaction driving force, molecular oxygen as the terminal oxidant and water as the only by-product are the salient features of this protocol. Authors have also studied the intramolecular kinetics and proposed mechanism of this protocol.
Downer et al. have introduced a new and applicable protocol for the intramolecular cyclization of thiobenzamides to benzothiazoles through the aryl radical cations as reactive intermediates (Scheme 50, Method 3).148 The protocol uses phenyliodine(III)bis(trifluoroacetate) (PIFA) in trifluoroethanol or cerium ammonium nitrate (CAN) in aqueous acetonitrile to enhance the cyclization within 30 min at room temperature, which afforded a moderate yield of the products.
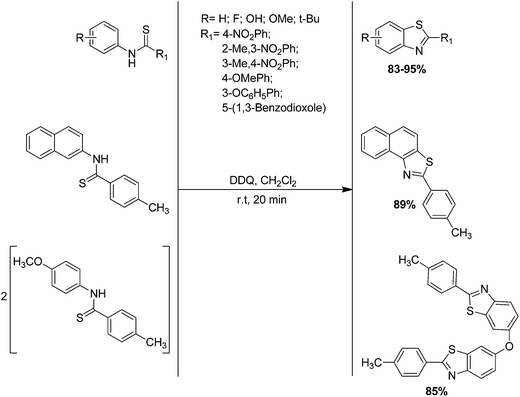
Bose et al. have introduced a new, practical and highly efficient route for the one step conversion of thioformanilides to 2-substituted benzothiazoles in high yield with selectivity by applying 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) as catalyst and dichloromethane (DCM) as a solvent at room temperature (Scheme 51).149 This protocol also follows the 1,5-homolytic radical cyclisation of thioyl radical to give 2-arylbenzothiazoles. 2-(p-Tolyl)naphthothiazole has been also synthesized in a moderate yield from 4-methyl-N-(naphthalen-2-yl)benzothioamide using this protocol.
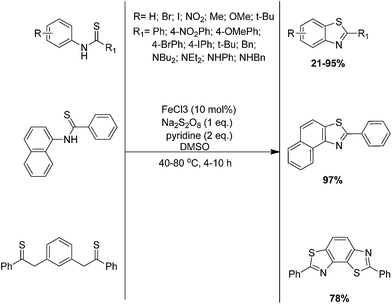
An efficient and transition metal catalysed intramolecular cyclization for the synthesis of benzothiazole derivatives using Na2S2O8 as the oxidant in dimethylsulfoxide (DMSO) with 10% FeCl3 as the catalyst and pyridine as the additive at 80 °C was developed by Wang and co-authors (Scheme 52).150 Preliminary mechanistic studies shows that the pyridine played a significant role in the high yields and selectivity of the products. According to the possible reaction mechanism, the thioyl radical intermediate was formed when the N-phenyl benzothioamide was oxidized by Fe(III), meanwhile Fe(III) was reduced to Fe(II). The thioyl radical intermediate was cyclised and oxidised in the presence of Na2S2O8 to give the product 2-phenyl benzothiazole.
Mu and co-authors have developed highly efficient method for the preparation of 2-benzoyl-benzothiazoles and 2-arylbenzothiazoles using manganese(III)triacetate (Mn(OAc)3) promoted radical cyclization of benzoylthioformanilides and arylthioformanilides under microwave irradiation (MWI) (Scheme 53).151 Here, Mn(OAc)3 is introduced as a new reagent to replace potassium ferricyanide (K3[Fe(CN)6]) or bromine (Br2), which are generally used for the radical cyclization of substituted thioformanilides.
Joyse et al. have investigated the synthesis of 2-aminobenzothiazoles from N-arylthioureas, via intramolecular C–S bond formation/C–H functionalization by employing an unusual co-catalytic Pd(PPh3)4/MnO2 system under an oxygen atmosphere at 80 °C, which afforded an excellent yield of the products (Scheme 54, Method 1).152 The chemoselective cyclization of substrates suggests that the reaction is very sensitive to steric effects at the ortho-position next to the C–H bond functionalization.
Jordan have developed a one-pot synthesis of 2-aminobenzothiazoles from either aryl isothiocyanates and amines or tetrabutylammonium thiocyanate and anilines in the presence of a stoichiometric amount of PhCH2N(Me)3(Br3), which afforded excellent yields of the products (Scheme 54, Method 2).153 Here, PhCH2N(Me)3(Br3) is a stable, electrophilic bromine source for the conversion of substituted arylthioureas to 2-aminobenzothiazoles under mild conditions in a variety of solvents with good yields.
Benedi and co-authors investigated a direct and efficient procedure for the synthesis of 2-amino- and 2-alkyl-benzothiazoles by cyclization of ortho-bromophenylthioureas and ortho-bromophenylthiamides in the presence of tris(dibenzylideneacetone)dipalladium(0) (Pd2(dba)3)/monophosphine catalyst (Scheme 55).154 Authors have also studied the effect of various ligands on the reaction, such as monophosphine (1,1′-bis(diphenylphosphino)ferrocene(dppf)), monophosphine (o-biphenylP(t-Bu)2) and n-BuPAd2, however highly hindered alkyl monophosphines have proven to be the most efficient ligand for this transformation.
Feng et al. have developed an efficient, economical, convenient and novel protocol for the synthesis of 2-substituted benzothiazoles from N′-substituted-N-(2-halophenyl)thioureas, O′-substituted-N-(2-halophenyl)carbamothioates, or N-(2-halophenyl)thioamides through a base-promoted intramolecular C–S bond coupling cyclization in dioxane without the use of any transition metal to afford high yields of the product (Scheme 56).155 To optimize the reaction conditions, the authors have studied the effect of various solvent and bases on this reaction, however the best results were obtained using Cs2CO3 as a base and dioxane as a solvent at 100–140 °C temperature for this reaction.
Jaseer et al. illustrated 1,1′-binaphthyl-2,2′-diamine (BINAM)–Cu(II) complex as a competent catalyst for the preparation of a broad range of 2-aryl/alkyl-substituted benzothiazoles by intramolecular C–S bond forming cyclization of poorly reactive N-(2-chlorophenyl)benzo or alkyl-thioamide under mild reaction conditions in the presence of acetonitrile at 82–110 °C for 27–96 h (Scheme 57, Method 1).156
A regioselective intramolecular C–S bond formation was indicated by Sahoo and colleagues during the formation of 2-aminobenzothiazoles from ortho-halothioureas using both Cu(I) and Pd(II) transition metal as a catalyst (Scheme 57, Method 2).157 Authors have noted that Cu preferred a dehalogenative route and Pd preferred predominantly C–H activation way for the creation of 2-aminobenzothiazoles. Here, Pd favoured C–H activation and Cu was unreactive in the absence of 2-chloro and 2-fluoro groups. However, for 2-bromo and 2-iodo aryl thioureas, identical selectivity was observed for both Cu- and Pd-catalyzed methods. A wide range of substituted derivatives were developed by this protocol which shows its wide synthetic utility.
Saha and colleagues have found a simple, general and efficient synthesis of substituted 2-aminobenzothiazoles via intramolecular cyclization of ortho-bromoaryl derivatives using copper(II)oxide nanoparticles as a catalyst in dimethyl sulfoxide (DMSO) solvent without additional external chelating ligands which affords high yields of the products (Scheme 57, Method 3).158 It is a heterogeneous process so the catalyst can be recovered and recycled without the loss of activity and selectivity of this methodology.
Hutchinson and colleagues have presented the regiospecific synthesis of a library of substituted 2-arylbenzothiazoles in the presence of sodium hydride (NaH) and N-methyl-2-pyrrolidone (NMP) at 140 °C from ortho-bromothiobenzanildes in excellent yield (Scheme 57, Method 4).159 In this method a bromine atom, ortho to the anilido nitrogen is used to direct a regiospecific cyclisation but in the absence of the bromine atom, a mixture of regioisomers is obtained. This protocol is applicable to the synthesis of 2-arylbenzothiazoles which has both electron-withdrawing and electron-donating substituents on the aryl ring.
Pal et al. have found a new and easy protocol for the preparation of 2-phenylbenzothiazole from benzanilide by direct thiation under microwave irradiation (MWI) in the presence of catalytic amounts of iodine (I2), which afforded moderate to good yields (Scheme 58, Method 1a and b).160 This reaction was carried out in various solvents and also under solvent-free conditions but the best result was obtained using dimethylformamide (DMF) as a solvent. This method is considered to be of wide synthetic utility because a variety of the benzoates of aromatic amines were employed with unsubstituted ortho-positions in this protocol. Experimental addition of a small amount of formic acid (HCOOH) gave increasing yields, although the reaction using formic acid as solvent was unsuccessful. For this reaction, the authors have discussed two probable mechanism pathways. The first may involve [4 + 2] cycloaddition of the iminol intermediate tautomer of benzoate and second may proceed via electrophilic attack of sulfur at C-2 of benzoate, as in the mechanism of the Willgerodt–Kindler reaction.
An efficient and economical access to substituted benzothiazoles has been introduced by Ma et al. using copper-catalyzed (CuI/L-proline) coupling of ortho-haloanilides with metal sulphides (Na2S·9H2O2) in N,N-dimethylformamide (DMF) as a solvent with excellent yields (Scheme 58, Method 2).161
Various ortho-bromobenzanilides were successfully converted into the corresponding benzothiazoles using a novel protocol, in which C–S bond formation occurred with the mercaptopropionate surrogate with pd/xantphos catalytic system followed by sequential deprotection and condensation, as demonstrated by Mase and co-authors (Scheme 58, Method 3).162 As a preliminary study for the preparation of sulfide, authors explored the reaction of ortho-bromoacetanilide with a mercaptopropionate surrogate catalysed by the Pd2(dba)3/xantphos system to afford the corresponding sulfide in good yield. This was further treated with sodium ethoxide (NaOEt) in ethanol (EtOH) at room temperature to procedure the sodium thiolate, which was further refluxed to give 2-methylbenzothiazole in 82% yield.
Copper-catalyzed double thiolation reaction of 1,4-dihalides with sulfides have been suggested by Li et al. for selective synthesis of 2-trifluoromethyl benzothiazoles (Scheme 59).163 The thiolation annulation of a variety of N-(2-haloaryl)trifluoroacetimidoyl chlorides by using CuI with sodium hydrosulfide hydrate (NaHS) and potassium phosphate (K3PO4), which afforded 2-trifluoromethylbenzothiazoles in moderate to good yields.
Murru and co-workers have developed the direct synthesis of arylthiobenzothiazoles via Cu(I) catalysed sequential intra- and intermolecular S-arylations in the presence of dimethyl sulfoxide (DMSO) as a solvent (Scheme 60).164 This protocol is superior to all reported methods for the preparation of the arylthiobenzothiazole due to low catalyst loading, an inexpensive metal catalyst and ligand, lower reaction temperature and shorter reaction time.
Jayakumar et al. have examined the reaction of ortho-aminothiophenol with various substituted 1,3-diazabuta-1,3-dienes(4-tertiaryamino-4-methylthio-1,3-diazabuta-1,3-dienes) under reflux conditions in dry toluene to afford N-benzothiazol-2-yl-N′-aryl benzamidines in good yields (Scheme 61).165
Shi et al. have determined a novel reductive cyclization of bis-(2-benzalaminophenyl)disulfide promoted by a titanium tetrachloride (TiCl4)/samarium (Sm) system using tetrahydrofuran (THF) as a solvent for the synthesis of 2-arylbenzothiazole derivatives in good yields (Scheme 62).166 Low-valent titanium was prepared from titanium tetrachloride and samarium powder. Various low-valent titanium reagent systems were surveyed by the authors. According to the results, TiCl4/Sm shows the best activity as a reductive reagent among those surveyed. Moreover, investigation of the reaction conditions disclosed that a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio of TiCl4 and Sm at 40 °C gave better results than those having the other reaction conditions. The advantages of this new method are easily accessible starting materials, short reaction time and moderate to good yields. Here, Bis-(2-benzalaminophenyl)disulfide was reduced by Ti(0), then the nucleophilic attack of the S− negative ion on C
2 ratio of TiCl4 and Sm at 40 °C gave better results than those having the other reaction conditions. The advantages of this new method are easily accessible starting materials, short reaction time and moderate to good yields. Here, Bis-(2-benzalaminophenyl)disulfide was reduced by Ti(0), then the nucleophilic attack of the S− negative ion on C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N group to give a cyclized intermediate and after that aromatization gives the expected product 2-arylbenzothiazole.
N group to give a cyclized intermediate and after that aromatization gives the expected product 2-arylbenzothiazole.
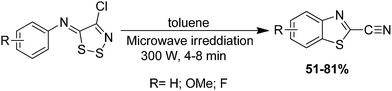
Beneteau et al. have noted that the 2-cyanobenzothiazoles can be prepared rapidly by applying microwave irradiation (MWI) (300 W) on neat N-arylimino-1,2,3-dithiazoles in screw capped glass vials for 4 to 8 min with good yield (Scheme 63).167 Here, no reaction was observed when the irradiations were carried out in an open vessel. Comparison of the preparation of 2-cyanobenzothiazoles by thermolysis and MWI was recorded by authors. However, they concluded that the best procedure for the preparation of 2-cyanobenzothiazoles is under microwave irradiation, which allows a striking reduction in reaction time.
Piscitelli et al. have developed solid supported synthesis of various substituted 2-aminobenzothiazole by using resin bounded isothiocyanate and various aniline derivatives (Scheme 64).168 The resin bounded isothiocyanates were converted to N-acyl or N-phenyl thioureas which cyclized to 2-acyl aminobenzothiazole by the treatment of 6 equiv. of bromine in acetic acid. Finally the desired compounds were obtained by treatment of 2-acyl aminobenzothiazole with 4% hydrazine monohydrate in ethanol (EtOH).
2.3 Using bromine as a catalyst
Schneider and colleagues have developed an efficient protocol for the preparation of 2,6-diamino tetrahydrobenzothiazole from 4-acetamidocyclohexane by brominating it in acetic acid (AcOH) and immediately treated it with thiourea to give the acetamido thiazole derivative in good yield (Scheme 65).169Singh and co-workers have synthesized the novel 2-amino-7-chloro-6-fluorobenzothiazole from 3-chloro-4-fluoroaniline and potassium thiocyanate (KSCN) in the presence of bromine (Br2) in glacial acetic acid (gla. CH3COOH/Br2) followed by basification with ammonia using the standard procedure (Scheme 66, Method 2).171
A series of 2-amino-substituted benzothiazoles were synthesized from the various substituted anilines by Himaja et al. utilizing potassium thiocyanate (KSCN) in the presence of bromine/glacial acetic acid (gla. CH3COOH/Br2) (Scheme 66, Method 3).171
Synthesis of various novel derivatives of 2-substituted-benzothiazole using potassium thiocyanate (KSCN) in the presence of gla. acetic acid and different substituted anilines have reported by Malik et al. (Scheme 66, Method 4).172
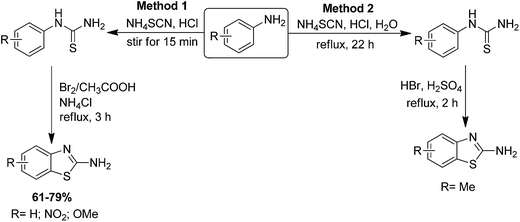
Alang and colleagues have shown the synthesis of a new derivative, 2-amino-6-methylbenzothiazole, in two steps by the reaction of para-toluidine with ammonium thiocyanate (NH4SCN) refluxing in HCl/H2O for 22 h which afforded para-tolylthiourea in excellent yield. This was further reacted with HBr and H2SO4 to produce 2-amino-6-methylbenzothiazole (Scheme 67, Method 2).174
2.4 Using Lawesson’s reagent and K3Fe(CN)6
A new library of 2-(4-aminophenyl)benzothiazoles were synthesized by Shi et al. using Lawesson’s reagent in hexamethylphosphoramide (HMPA) or chlorobenzene (ClC6H5) followed by the treatment of potassium ferricyanide (K3[Fe(CN)6]) in aq. sodium hydroxide at 80–90 °C, which afforded excellent yield of the products (Scheme 69a).176Authors have also synthesized a new library of benzothiazole using suitable methods for each benzothiazole moiety (Scheme 69b).176
Different methods were used to synthesize different derivatives of mono- and difluorinated 2-(4-nitro-3-substituted phenyl)benzothiazoles by Hutchinson et al. (Scheme 70a–d).12
Huang et al. have reported a new benzothiazole derivative using the same method as Shi et al. (Scheme 71, Method 1).177
Lyon and co-workers have also synthesized substituted 2-phenylbenzothiazole using the method discussed above (Scheme 71, Method 2).178
Some other derivatives of 2-(6-substituted-benzothiazol-2-yl)phenol were synthesized by Wang and colleagues from various substituted anilines and ortho-hydroxybenzoicacid through the method described above (Scheme 71, Method 3).179
Kashiyama and co-authors have synthesized a series of 2-phenyl-1,3-benzothiazoles from N-phenylbenzamide using Lawesson’s reagent and Potassium ferricyanide (K3[Fe(CN)6]) (Scheme 71, Method 4).28
2.5 From coumarin
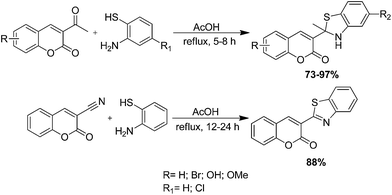
The synthesis of a series of coumarin derivatives containing a 2-methylbenzothiazoline moiety and related compounds have been prepared by Khoobi and co-workers via the reaction of 3-acetylcoumarins with ortho-aminothiophenol derivatives in the presence of acetic acid (AcOH) under reflux conditions (Scheme 72).180 Some of these coumarin derivatives have been synthesized by the reaction of 3-cyanocoumarin with ortho-aminothiophenol using the same reaction conditions.Khoobi et al. have introduced a rapid, simple and convenient microwave-assisted protocol for the synthesis of 3-substituted coumarin derivatives by the reaction of 3-acetyl and 3-cyanocoumarins with ortho-aminothiophenol derivatives in the presence of acetic acid (AcOH) or catalytic amounts of HPMo in good to excellent isolated yields (Scheme 73).181 Generality, simplicity, easy work up, clean reactions and improved yields are the beneficial points of this methodology.
2.6 Miscellaneous
Deohate have investigated a novel series of {4-[4-(6-substituted-benzothiazol-2-yl-amino)-3-carboxy-benzyl]-2-carboxyphenyl}-6-substituted-benzothiazol-2-ylamines, which were synthesized by oxidative cyclization of 1-{4-[4-(3-aryl thiocarbamido)-3-carboxy-benzyl]-2-carboxy-phenyl}-3-aryl thiocarbamides refluxing in a solution of bromine in chloroform (Br2 in CHCl3) followed by basification with dilute ammonium hydroxide (NH4OH) solution (Scheme 74).182 1-{4-[4-(3-Aryl thiocarbamido)-3-carboxy-benzyl]-2-carboxy-phenyl}-3-aryl thiocarbamides were prepared by the collaboration of diverse aryl isothiocyanates with 4,4′-methylenebis-(anthranilicacid).The first practical one-pot methodology for the synthesis of 4-hydroxy-6-(2-amino-2-carboxyethyl)benzothiazole and 4-hydroxy-7-(2-amino-2-carboxyethyl)benzothiazole and their corresponding 2-carboxy-derivatives were reported by Greco et al. involving sequential Zn2+-assisted biomimetic oxidation of L-dopa and L-cysteine, 5-S-cysteinyldopa or 2-S-cysteinyldopa (Scheme 75).183 In this protocol, the biogenetic generation of pheomelanins from L-tyrosine and L-cysteine occurs via tyrosinase catalyzed conversion to 5-S- and 2-S-cysteinyldopa conjugates and then 2-carboxy-6-(2-amino-2-carboxyethyl)benzothiazole and 2-carboxy-7-(2-amino-2-carboxyethyl)benzothiazole are generated by pigment degradation.
A convenient and efficient 4-step synthesis of 2-methyl-5-(methylthio)benzothiazole, which uses inexpensive reagents and starting materials, has been demonstrated by Lynch and colleagues (Scheme 76).184 It is an efficient, scalable, clean and convenient methodology for the synthesis of substituted benzothiazoles. The preparation of 4-chloro-2-nitrobenzenesulfonylchloride from 4-chloro-3-nitrobenznesulfonic acid and sodium salt was performed using phosphorous oxychloride (POCl3) and sulfolane(2,3,4,5-tetrahydrothiophene-1,1-dioxide) according to a general procedure described by Fujita et al. Then, reduction of the sulfonyl chloride was performed with 55% hydriodic acid and a catalytic amount of potassium iodide (KI) and sodium metabisulfite (Na2S2O5) using an organic synthesis procedure gave bis-(4-chloro-3-nitrophenyl)disulfide. The disulfide was reduced to the thiol with sodium sulfide (Na2S) and then treated with dimethyl sulphate (SO2(OMe)2) according to the procedure of Bourquin et al. to give the required benzothiazole. The one-pot conversion of l-chloro-4-(methylthio)-2-nitrobenzeneinto 2-methyl-5-(methylthio)benzothiazole is the key step in this process.
3. Conclusion
Benzothiazole and its derivatives possess a wide spectrum of biological and industrial applications. A number of procedures for their synthesis have been developed because of the development of green and environmentally friendly chemical processes due to their cost-effectiveness, better selectivity, operational simplicity and high yield of the products. It is interesting to note that the condensation of ortho-amino thiophenols with various acids/aldehydes/ketones/nitriles/esters has drawn more attention for the synthesis of benzothiazole derivatives. However, 2-amino benzothiazoles were efficiently synthesised from the reaction between various aromatic amines and ammonium thiocyanate/sodium thiocyanate/potassium thiocyanate using bromine. Here, we have made the efforts to compile most of these methods, which are reported recently. Thus, this work represents a valuable complement to the overall development for the synthesis of benzothiazole derivatives by various synthetic routes.Acknowledgements
The authors are thankful to the Department of Chemistry, Gujarat University, Ahmedabad, for providing the necessary facilities and also thankful to UGC-Info net & INFLIBNET Gujarat University for providing e-source facilities. One of the authors, R.H.V. is thankful to UGC-BSR (F.7-74/2007 (BSR)) for financial assistance.References
- G. P. Gunawardana, S. Kohmoto, S. P. Gunasekera, O. J. McConnell and F. E. Koehn, J. Am. Chem. Soc., 1988, 110, 4856–4858 CrossRef CAS.
- G. P. Gunawardana, F. E. Koehn, A. Y. Lee, J. Clardy, H. Y. He and D. J. Faulkner, J. Org. Chem., 1992, 57, 1523–1526 CrossRef CAS.
- A. R. Carroll and P. J. Scheuer, J. Org. Chem., 1990, 55, 4426–4431 CrossRef CAS.
- M. N. Bhoi, M. A. Borad and H. D. Patel, Synth. Commun., 2014, 44, 2427–2457 CrossRef CAS.
- M. B. Harriet, F. Bret and B. Paul, Drugs, 1996, 52, 549–563 CrossRef PubMed.
- Y. Hu and J. B. MacMillan, Org. Lett., 2011, 13, 6580–6583 CrossRef CAS PubMed.
- S. Gupta, N. Ajmera, N. Gautam, R. Sharma and D. Gautam, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2009, 48, 853–858 Search PubMed.
- Y. Murthi and D. Pathak, J. Pharm. Res., 2008, 7, 153–155 Search PubMed.
- M. A. Mahran, S. William, F. Ramzy and A. M. Sembel, Molecules, 2007, 12, 622–633 CrossRef CAS.
- P. Palmer, R. Trigg and J. Warrington, J. Med. Chem., 1971, 14, 1228–1230 CrossRef CAS.
- S. H. L. Kok, R. Gambari, C. H. Chui, M. C. W. Yuen, E. Lin, R. S. M. Wong, F. Y. Lau, G. Y. M. Cheng, W. S. Lam and S. H. Chan, Bioorg. Med. Chem., 2008, 16, 3626–3631 CrossRef CAS PubMed.
- I. Hutchinson, M.-S. Chua, H. L. Browne, V. Trapani, T. D. Bradshaw, A. D. Westwell and M. F. Stevens, J. Med. Chem., 2001, 44, 1446–1455 CrossRef CAS PubMed.
- I. Ćaleta, M. Grdiša, D. Mrvoš-Sermek, M. Cetina, V. Tralić-Kulenović, K. Pavelić and G. Karminski-Zamola, Il Farmaco, 2004, 59, 297–305 CrossRef PubMed.
- G. Wells, T. D. Bradshaw, P. Diana, A. Seaton, D.-F. Shi, A. D. Westwell and M. F. Stevens, Bioorg. Med. Chem. Lett., 2000, 10, 513–515 CrossRef CAS.
- Y. Heo, Y. S. Song, B. T. Kim and J.-N. Heo, Tetrahedron Lett., 2006, 47, 3091–3094 CrossRef CAS PubMed.
- S. T. Huang, I. J. Hsei and C. Chen, Bioorg. Med. Chem., 2006, 14, 6106–6119 CrossRef CAS PubMed.
- G. Sreenivasa, E. Jayachandran, B. Shivakumar, K. Jayaraj and M. Kumar Vijay, Arch. Pharm. Sci. Res., 2009, 1, 150–157 CAS.
- X. Su, N. Vicker, D. Ganeshapillai, A. Smith, A. Purohit, M. J. Reed and B. V. Potter, Mol. Cell. Endocrinol., 2006, 248, 214–217 CrossRef CAS PubMed.
- H. Moreno-Díaz, R. Villalobos-Molina, R. Ortiz-Andrade, D. Díaz-Coutiño, J. L. Medina-Franco, S. P. Webster, M. Binnie, S. Estrada-Soto, M. Ibarra-Barajas and I. León-Rivera, Bioorg. Med. Chem. Lett., 2008, 18, 2871–2877 CrossRef PubMed.
- P. Palmer, R. Trigg and J. Warrington, J. Med. Chem., 1971, 14, 248–251 CrossRef CAS.
- J. Das, R. V. Moquin, J. Lin, C. Liu, A. M. Doweyko, H. F. DeFex, Q. Fang, S. Pang, S. Pitt and D. R. Shen, Bioorg. Med. Chem. Lett., 2003, 13, 2587–2590 CrossRef CAS.
- T. Bradshaw and A. Westwell, Curr. Med. Chem., 2004, 11, 1009–1021 CrossRef CAS.
- M. Yoshida, I. Hayakawa, N. Hayashi, T. Agatsuma, Y. Oda, F. Tanzawa, S. Iwasaki, K. Koyama, H. Furukawa and S. Kurakata, Bioorg. Med. Chem. Lett., 2005, 15, 3328–3332 CrossRef CAS PubMed.
- C. J. Lion, C. S. Matthews, G. Wells, T. D. Bradshaw, M. F. Stevens and A. D. Westwell, Bioorg. Med. Chem. Lett., 2006, 16, 5005–5008 CrossRef CAS PubMed.
- V. Bénéteau, T. Besson, J. Guillard, S. Léonce and B. Pfeiffer, Eur. J. Med. Chem., 1999, 34, 1053–1060 CrossRef.
- I. Hutchinson, T. D. Bradshaw, C. S. Matthews, M. F. Stevens and A. D. Westwell, Bioorg. Med. Chem. Lett., 2003, 13, 471–474 CrossRef CAS.
- I. H. Hall, N. J. Peaty, J. R. Henry, J. Easmon, G. Heinisch and G. Pürstinger, Arch. Pharm., 1999, 332, 115–123 CrossRef CAS.
- E. Kashiyama, I. Hutchinson, M.-S. Chua, S. F. Stinson, L. R. Phillips, G. Kaur, E. A. Sausville, T. D. Bradshaw, A. D. Westwell and M. F. Stevens, J. Med. Chem., 1999, 42, 4172–4184 CrossRef CAS PubMed.
- J. Neres, M. L. Brewer, L. Ratier, H. Botti, A. Buschiazzo, P. N. Edwards, P. N. Mortenson, M. H. Charlton, P. M. Alzari and A. C. Frasch, Bioorg. Med. Chem. Lett., 2009, 19, 589–596 CrossRef CAS PubMed.
- P. Vicini, A. Geronikaki, M. Incerti, B. Busonera, G. Poni, C. A. Cabras and P. La Colla, Bioorg. Med. Chem., 2003, 11, 4785–4789 CrossRef CAS.
- S. R. Nagarajan, G. A. De Crescenzo, D. P. Getman, H.-F. Lu, J. A. Sikorski, J. L. Walker, J. J. McDonald, K. A. Houseman, G. P. Kocan and N. Kishore, Bioorg. Med. Chem., 2003, 11, 4769–4777 CrossRef CAS PubMed.
- C. J. Paget, K. Kisner, R. L. Stone and D. C. DeLong, J. Med. Chem., 1969, 12, 1016–1018 CrossRef CAS.
- R. J. Alaimo, S. S. Pelosi and R. Freedman, J. Pharm. Sci., 1978, 67, 281–282 CrossRef CAS.
- D. D. Sogn, R. Evans, G. M. Shepherd, T. B. Casale, J. Condemi, P. A. Greenberger, P. F. Kohler, A. Saxon, R. J. Summers and P. P. VanArsdel, Arch. Intern. Med., 1992, 152, 1025–1032 CrossRef CAS.
- K. Mistry and K. Desai, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2006, 45, 1762 Search PubMed.
- M. Singh, S. K. Singh, M. Gangwar, G. Nath and S. K. Singh, RSC Adv., 2014, 4, 19013–19023 RSC.
- D. Cressier, C. Prouillac, P. Hernandez, C. Amourette, M. Diserbo, C. Lion and G. Rima, Bioorg. Med. Chem., 2009, 17, 5275–5284 CrossRef CAS PubMed.
- A. Benazzouz, T. Boraud, P. Dubédat, A. Boireau, J.-M. Stutzmann and C. Gross, Eur. J. Pharmacol., 1995, 284, 299–307 CrossRef CAS.
- P. Jimonet, F. Audiau, M. Barreau, J.-C. Blanchard, A. Boireau, Y. Bour, M.-A. Coléno, A. Doble, G. Doerflinger and C. Do Huu, J. Med. Chem., 1999, 42, 2828–2843 CrossRef CAS PubMed.
- I. Hutchinson, S. A. Jennings, B. R. Vishnuvajjala, A. D. Westwell and M. F. Stevens, J. Med. Chem., 2002, 45, 744–747 CrossRef CAS PubMed.
- M. A. El-Sherbeny, Arzneim. Forsch., 2000, 50, 848–853 CAS.
- P. Naik, S. Pandeya and A. Pandey, Indian J. Physiol. Pharmacol., 1996, 40, 189–190 CAS.
- D. Dogruer, S. Unlu, M. Sahin and E. Yesilada, Il Farmaco, 1998, 53, 80 CrossRef CAS.
- A. A. Chavan and N. R. Pai, Molecules, 2007, 12, 2467–2477 CrossRef CAS.
- Y. Liu, Y. Wang, G. Dong, Y. Zhang, S. Wu, Z. Miao, J. Yao, W. Zhang and C. Sheng, Med. Chem. Commun., 2013, 4, 1551–1561 RSC.
- R. A. Tapia, Y. Prieto, F. Pautet, M. Domard, M. E. Sarciron, N. Walchshofer and H. Fillion, Eur. J. Org. Chem., 2002, 2002, 4005–4010 CrossRef.
- S. Nadeem, A. Rana, S. A. Khan, S. E. Haque, M. S. Alam and W. Ahsana, Acta Chim. Slov., 2009, 56, 462–469 Search PubMed.
- R. Danzeisen, B. Schwalenstoecker, F. Gillardon, E. Buerger, V. Krzykalla, K. Klinder, L. Schild, B. Hengerer, A. C. Ludolph and C. Dorner-Ciossek, J. Pharmacol. Exp. Ther., 2006, 316, 189–199 CrossRef CAS PubMed.
- B. Rajeeva, N. Srinivasulu and S. Shantakumar, E-J. Chem., 2009, 6, 775–779 CrossRef CAS PubMed.
- B. Gurupadayya, Y. Manohara and M. Gopal, Indian J. Heterocycl. Chem., 2006, 15, 113–116 Search PubMed.
- J. M. Bergman, P. J. Coleman, C. D. Cox, G. D. Hartman, C. Lindsley, S. P. Mercer, A. J. Roecker and D. B. Whitman, PCT Int. Appl. WO 2006127550, 2006.
- B. L. Mylari, E. R. Larson, T. A. Beyer, W. J. Zembrowski, C. E. Aldinger, M. F. Dee, T. W. Siegel and D. H. Singleton, J. Med. Chem., 1991, 34, 108–122 CrossRef CAS.
- G. Sato, T. Chimoto, T. Aoki, S. Hosokawa, S. Sumigama, K. Tsukidate and F. Sagami, J. Toxicol. Sci., 1999, 24, 165–175 CrossRef CAS.
- K. Yoshino, T. Kohno, T. Uno, T. Morita and G. Tsukamoto, J. Med. Chem., 1986, 29, 820–825 CrossRef CAS.
- S. J. Hays, M. J. Rice, D. F. Ortwine, G. Johnson, R. D. Schwarz, D. K. Boyd, L. F. Copeland, M. G. Vartanian and P. A. Boxer, J. Pharm. Sci., 1994, 83, 1425–1432 CrossRef CAS.
- C. Lau, C. Dufresne, Y. Gareau, R. Zamboni, M. Labelle, R. Young, K. Metters, C. Rochette, N. Sawyer and D. Slipetz, Bioorg. Med. Chem. Lett., 1995, 5, 1615–1620 CrossRef CAS.
- T. Itoh and T. Mase, Org. Lett., 2007, 9, 3687–3689 CrossRef CAS PubMed.
- I. Petkov, T. Deligeorgiev, P. Markov, M. Evstatiev and S. Fakirov, Polym. Degrad. Stab., 1991, 33, 53–66 CrossRef CAS.
- K. Yamamoto, M. Fujita, K. Tabashi, Y. Kawashima, E. Kato, M. Oya, T. Iso and J. Iwao, J. Med. Chem., 1988, 31, 919–930 CrossRef CAS.
- B. Lara, L. Gandía, A. Torres, R. Olivares, R. Martínez-Sierra, A. G. García and M. G. López, Eur. J. Pharmacol., 1997, 325, 109–119 CrossRef CAS.
- J. Jia, M. Cui, J. Dai, X. Wang, Y. S. Ding, H. Jia and B. Liu, Med. Chem. Commun., 2014, 5, 153–158 RSC.
- F. Shah, Y. Wu, J. Gut, Y. Pedduri, J. Legac, P. J. Rosenthal and M. A. Avery, Med. Chem. Commun., 2011, 2, 1201–1207 RSC.
- C. A. Mathis, Y. Wang, D. P. Holt, G.-F. Huang, M. L. Debnath and W. E. Klunk, J. Med. Chem., 2003, 46, 2740–2754 CrossRef CAS PubMed.
- D. Alagille, R. M. Baldwin and G. D. Tamagnan, Tetrahedron Lett., 2005, 46, 1349–1351 CrossRef CAS PubMed.
- M.-Q. Zheng, D.-Z. Yin, J.-P. Qiao, L. Zhang and Y.-X. Wang, J. Fluorine Chem., 2008, 129, 210–216 CrossRef CAS PubMed.
- K. Serdons, T. Verduyckt, D. Vanderghinste, J. Cleynhens, P. Borghgraef, P. Vermaelen, C. Terwinghe, F. V. Leuven, K. V. Laere and H. Kung, Bioorg. Med. Chem. Lett., 2009, 19, 602–605 CrossRef CAS PubMed.
- A. K. Chakraborti, S. Rudrawar, G. Kaur and L. Sharma, Synlett, 2004, 1533–1536 CrossRef CAS PubMed.
- K. Yoshino, K. Goto, K. Yoshiizumi, T. Morita and G. Tsukamoto, J. Med. Chem., 1990, 33, 2192–2196 CrossRef CAS.
- H. Y. Guo, J. C. Li and Y. L. Shang, Chin. Chem. Lett., 2009, 20, 1408–1410 CrossRef CAS PubMed.
- C. G. Mortimer, G. Wells, J.-P. Crochard, E. L. Stone, T. D. Bradshaw, M. F. Stevens and A. D. Westwell, J. Med. Chem., 2006, 49, 179–185 CrossRef CAS PubMed.
- L. Sattler, F. Zerban, G. Clark and C.-C. Chu, J. Am. Chem. Soc., 1951, 73, 5908–5910 CrossRef CAS.
- A. G. Green and A. G. Perkin, J. Chem. Soc., 1903, 70, 1207 Search PubMed.
- B. Maleki and H. Salehabadi, Eur. J. Chem., 2010, 1, 377–380 CrossRef CAS.
- R. M. Batista, S. P. Costa and M. M. M. Raposo, Tetrahedron Lett., 2004, 45, 2825–2828 CrossRef CAS PubMed.
- C. Praveen, A. Nandakumar, P. Dheenkumar, D. Muralidharan and P. Perumal, J. Chem. Sci., 2012, 124, 609–624 CrossRef CAS.
- A. Dandia, B. Rani, M. Saha and I. Gupta, Phosphorus, Sulfur Silicon Relat. Elem., 1997, 130, 217–227 CrossRef CAS.
- S. Paul, M. Gupta and R. Gupta, Synth. Commun., 2002, 32, 3541–3547 CrossRef CAS PubMed.
- S. V. Nalage, S. V. Bhosale, D. S. Bhosale and W. N. Jadhav, Chin. Chem. Lett., 2010, 21, 790–793 CrossRef CAS PubMed.
- P. S. Chandrachood, D. R. Garud, T. V. Gadakari, R. C. Torane, N. R. Deshpande and R. V. Kashalkar, Acta Chim. Slov., 2011, 58, 367–371 CAS.
- F. Matloubi Moghaddam, G. Rezanejade Bardajee, H. Ismaili and S. Maryam Dokht Taimoory, Synth. Commun., 2006, 36, 2543–2548 CrossRef.
- A. A. Weekes, M. C. Dix, M. C. Bagley and A. D. Westwell, Synth. Commun., 2010, 40, 3027–3032 CrossRef CAS.
- A. J. Blacker, M. M. Farah, M. I. Hall, S. P. Marsden, O. Saidi and J. M. Williams, Org. Lett., 2009, 11, 2039–2042 CrossRef CAS PubMed.
- K. Bahrami, M. M. Khodaei and F. Naali, J. Org. Chem., 2008, 73, 6835–6837 CrossRef CAS PubMed.
- S. S. Patil and V. D. Bobade, Synth. Commun., 2010, 40, 206–212 CrossRef CAS.
- M. T. Bogert and B. Naiman, J. Am. Chem. Soc., 1935, 57, 1529–1533 CrossRef CAS.
- V. S. Padalkar, B. N. Borse, V. D. Gupta, K. R. Phatangare, V. S. Patil, P. G. Umape and N. Sekar, Arabian J. Chem., 2011 DOI:10.1016/j.arabjc.2011.12.006.
- B. Maleki, H. Salehabadi and M. Khodaverdian Moghaddam, Acta Chim. Slov., 2010, 57, 741–745 CAS.
- A. Shokrolahi, A. Zali and M. Mahdavi, Phosphorus, Sulfur Silicon Relat. Elem., 2012, 187, 535–543 CrossRef CAS.
- M. Abdollahi-Alibeik and S. Poorirani, Phosphorus, Sulfur Silicon Relat. Elem., 2009, 184, 3182–3190 CrossRef CAS.
- A. Ben-Alloum, S. Bakkas and M. Soufiaoui, Tetrahedron Lett., 1997, 38, 6395–6396 CrossRef CAS.
- Y. Kawashita, C. Ueba and M. Hayashi, Tetrahedron Lett., 2006, 47, 4231–4233 CrossRef CAS PubMed.
- A. Kumar, R. A. Maurya and P. Ahmad, J. Comb. Chem., 2009, 11, 198–201 CrossRef CAS PubMed.
- T. G. Deligeorgiev, S. Kaloyanova, A. Vasilev and J. J. Vaquero, Phosphorus, Sulfur Silicon Relat. Elem., 2010, 185, 2292–2302 CrossRef CAS.
- C. L. Lee, Y. Lam and S.-Y. Lee, Tetrahedron Lett., 2001, 42, 109–111 CrossRef CAS.
- S. Sadjadi and H. Sepehrian, Ultrason. Sonochem., 2011, 18, 480–483 CrossRef CAS PubMed.
- V. S. Padalkar, V. D. Gupta, K. R. Phatangare, V. S. Patil, P. G. Umape and N. Sekar, Green Chem. Lett. Rev., 2012, 5, 139–145 CrossRef CAS.
- Y. Q. Yuan and S.-R. Guo, Synth. Commun., 2011, 41, 2169–2177 CrossRef CAS.
- M. Kodomari, Y. Tamaru and T. Aoyama, Synth. Commun., 2004, 34, 3029–3036 CrossRef CAS PubMed.
- X. Fan, Y. Wang, Y. He, X. Zhang and J. Wang, Tetrahedron Lett., 2010, 51, 3493–3496 CrossRef CAS PubMed.
- X. Fan, Y. He, Y. Wang, Z. Xue, X. Zhang and J. Wang, Tetrahedron Lett., 2011, 52, 899–902 CrossRef CAS PubMed.
- U. R. Pratap, J. R. Mali, D. V. Jawale and R. A. Mane, Tetrahedron Lett., 2009, 50, 1352–1354 CrossRef CAS PubMed.
- Y. Riadi, R. Mamouni, R. Azzalou, M. E. Haddad, S. Routier, G. Guillaumet and S. Lazar, Tetrahedron Lett., 2011, 52, 3492–3495 CrossRef CAS PubMed.
- P. Bandyopadhyay, M. Sathe, S. Ponmariappan, A. Sharma, P. Sharma, A. Srivastava and M. Kaushik, Bioorg. Med. Chem. Lett., 2011, 21, 7306–7309 CrossRef CAS PubMed.
- A. Mokhir, K. Domasevich, N. Kent Dalley, X. Kou, N. Gerasimchuk and O. Gerasimchuk, Inorg. Chim. Acta, 1999, 284, 85–98 CrossRef CAS.
- M. C. Van Zandt, E. O. Sibley, E. E. McCann, K. J. Combs, B. Flam, D. R. Sawicki, A. Sabetta, A. Carrington, J. Sredy and E. Howard, Bioorg. Med. Chem., 2004, 12, 5661–5675 CrossRef CAS PubMed.
- S. Yadong, J. Huanfeng, W. Wanqing, Z. Wei and W. Xia, Org. Lett., 2013, 15, 1598–1601 CrossRef PubMed.
- Z. H. Khalil, A. S. Yanni, A. M. Gaber and S. A. Abdel-Mohsen, Phosphorus, Sulfur Silicon Relat. Elem., 2000, 166, 57–69 CrossRef CAS.
- G. Manfroni, F. Meschini, M. L. Barreca, P. Leyssen, A. Samuele, N. Iraci, S. Sabatini, S. Massari, G. Maga, J. Neyts and V. Cecchetti, Bioorg. Med. Chem., 2012, 20, 866–876 CrossRef CAS PubMed.
- A. Chandra Sheker Reddy, P. Shanthan Rao and R. Venkataratnam, Tetrahedron, 1997, 53, 5847–5854 CrossRef.
- G. Bastug, C. Eviolitte and I. E. Markó, Org. Lett., 2012, 14, 3502–3505 CrossRef CAS PubMed.
- H. J. Karlsson, M. H. Bergqvist, P. Lincoln and G. Westman, Bioorg. Med. Chem., 2004, 12, 2369–2384 CrossRef CAS PubMed.
- W. Huang and G.-F. Yang, Bioorg. Med. Chem., 2006, 14, 8280–8285 CrossRef CAS PubMed.
- H. Sharghi and O. Asemani, Synth. Commun., 2009, 39, 860–867 CrossRef CAS.
- I. Yildiz-Oren, I. Yalcin, E. Aki-Sener and N. Ucarturk, Eur. J. Med. Chem., 2004, 39, 291–298 CrossRef CAS PubMed.
- S. D. Gupta, H. P. Singh and N. Moorthy, Synth. Commun., 2007, 37, 4327–4329 CrossRef CAS.
- R. Abdul, G. Saloni and S. Shweta, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2008, 47, 601–605 Search PubMed.
- F. Ge, Z. Wang, W. Wan, W. Lu and J. Hao, Tetrahedron Lett., 2007, 48, 3251–3254 CrossRef CAS PubMed.
- L. Racane, M. Kralj, L. Suman, R. Stojkovic, V. Tralic-Kulenovic and G. Karminski-Zamola, Bioorg. Med. Chem., 2010, 18, 1038–1044 CrossRef CAS PubMed.
- K. R. Kumar, P. Satyanarayana and B. Srinivasa Reddy, E-J. Chem., 2012, 2013, 1–10 Search PubMed.
- R. N. Nadaf, S. A. Siddiqui, T. Daniel, R. J. Lahoti and K. V. Srinivasan, J. Mol. Catal. A: Chem., 2004, 214, 155–160 CrossRef CAS PubMed.
- H. J. Karlsson, M. H. Bergqvist, P. Lincoln and G. Westman, Bioorg. Med. Chem., 2004, 12, 2369–2384 CrossRef CAS PubMed.
- C. Wu, J. Wei, K. Gao and Y. Wang, Bioorg. Med. Chem., 2007, 15, 2789–2796 CrossRef CAS PubMed.
- Q. Ding, X.-G. Huang and J. Wu, J. Comb. Chem., 2009, 11, 1047–1049 CrossRef CAS PubMed.
- A. S. El-Sharief, Y. Ammar, M. Zahran and H. K. Sabet, Phosphorus, Sulfur Silicon Relat. Elem., 2004, 179, 267–275 CrossRef CAS.
- M. Ghorab, A. Osman, E. Noaman, H. Heiba and N. Zaher, Phosphorus, Sulfur Silicon Relat. Elem., 2006, 181, 1983–1996 CrossRef CAS.
- R. Cano, D. J. Ramón and M. Yus, J. Org. Chem., 2010, 76, 654–660 CrossRef PubMed.
- Q. Ding, X. He and J. Wu, J. Comb. Chem., 2009, 11, 587–591 CrossRef CAS PubMed.
- Y. J. Guo, R.-Y. Tang, P. Zhong and J. H. Li, Tetrahedron Lett., 2010, 51, 649–652 CrossRef CAS PubMed.
- Z. G. Le, J.-P. Xu, H. Y. Rao and M. Ying, J. Heterocycl. Chem., 2006, 43, 1123–1124 CrossRef CAS.
- S. Murru, P. Mondal, R. Yella and B. K. Patel, Eur. J. Org. Chem., 2009, 2009, 5406–5413 CrossRef.
- T. B. Nguyen, L. Ermolenko, W. A. Dean and A. Al-Mourabit, Org. Lett., 2012, 14, 5948–5951 CrossRef CAS PubMed.
- Y. Liao, H. Qi, S. Chen, P. Jiang, W. Zhou and G.-J. Deng, Org. Lett., 2012, 14, 6004–6007 CrossRef CAS PubMed.
- R. C. Elderfield and E. C. McClenachan, J. Am. Chem. Soc., 1960, 82, 1982–1988 CrossRef CAS.
- F. J. Kreysa, V. F. Maturi, J. J. Finn, J. J. McClarnon and F. Lombardo, J. Am. Chem. Soc., 1951, 73, 1155–1156 CrossRef CAS.
- W. J. Xue, Y. Q. Guo, F.-F. Gao, H. Z. Li and A. X. Wu, Org. Lett., 2013, 15, 890–893 CrossRef CAS PubMed.
- F. Wang, S. Cai, Z. Wang and C. Xi, Org. Lett., 2011, 13, 3202–3205 CrossRef CAS PubMed.
- L. Shi, X. Liu, H. Zhang, Y. Jiang and D. Ma, J. Org. Chem., 2011, 76, 4200–4204 CrossRef CAS PubMed.
- D. Ma, X. Lu, L. Shi, H. Zhang, Y. Jiang and X. Liu, Angew. Chem., Int. Ed., 2011, 50, 1118–1121 CrossRef CAS PubMed.
- U. Klar, B. Buchmann, W. Schwede, W. Skuballa, J. Hoffmann and R. B. Lichtner, Angew. Chem., Int. Ed., 2006, 45, 7942–7948 CrossRef CAS PubMed.
- H. Xiang, J. Qi, Q. He, M. Jiang, C. Yang and L. Deng, Org. Biomol. Chem., 2014, 12, 4633–4636 CAS.
- X. Zhou, L. Pan, L. Yu, Z. Wu, Z. Li and H. Xiang, RSC Adv., 2014, 4, 27775–27779 RSC.
- V. A. Ogurtsov, O. A. Rakitin, C. W. Rees and A. A. Smolentsev, Mendeleev Commun., 2003, 13, 50–51 CrossRef PubMed.
- D. Hein, R. J. Alheim and J. Leavitt, J. Am. Chem. Soc., 1957, 79, 427–429 CrossRef CAS.
- G. Melikyan, A. Avetisyan and J. Halgaš, Chem. Pap., 1992, 46, 109–112 CAS.
- Y. p. Zhu, F. c. Jia, M. c. Liu and A. x. Wu, Org. Lett., 2012, 14, 4414–4417 CrossRef CAS PubMed.
- V. Rey, S. M. Soria-Castro, J. E. Argüello and A. B. Peñéñory, Tetrahedron Lett., 2009, 50, 4720–4723 CrossRef CAS PubMed.
- Y. Cheng, J. Yang, Y. Qu and P. Li, Org. Lett., 2011, 14, 98–101 CrossRef PubMed.
- N. K. Downer-Riley and Y. A. Jackson, Tetrahedron, 2008, 64, 7741–7744 CrossRef CAS PubMed.
- D. S. Bose and M. Idrees, Tetrahedron Lett., 2007, 48, 669–672 CrossRef CAS PubMed.
- H. Wang, L. Wang, J. Shang, X. Li, H. Wang, J. Gui and A. Lei, Chem. Commun., 2012, 48, 76–78 RSC.
- X. J. Mu, J. P. Zou, R. S. Zeng and J. C. Wu, Tetrahedron Lett., 2005, 46, 4345–4347 CrossRef CAS PubMed.
- L. L. Joyce and R. A. Batey, Org. Lett., 2009, 11, 2792–2795 CrossRef CAS PubMed.
- A. D. Jordan, C. Luo and A. B. Reitz, J. Org. Chem., 2003, 68, 8693–8696 CrossRef CAS PubMed.
- C. Benedí, F. Bravo, P. Uriz, E. Fernández, C. Claver and S. Castillón, Tetrahedron Lett., 2003, 44, 6073–6077 CrossRef.
- E. Feng, H. Huang, Y. Zhou, D. Ye, H. Jiang and H. Liu, J. Comb. Chem., 2010, 12, 422–429 CrossRef CAS PubMed.
- E. Jaseer, D. Prasad, A. Dandapat and G. Sekar, Tetrahedron Lett., 2010, 51, 5009–5012 CrossRef CAS PubMed.
- S. K. Sahoo, A. Banerjee, S. Chakraborty and B. K. Patel, ACS Catal., 2012, 2, 544–551 CrossRef CAS.
- P. Saha, T. Ramana, N. Purkait, M. A. Ali, R. Paul and T. Punniyamurthy, J. Org. Chem., 2009, 74, 8719–8725 CrossRef CAS PubMed.
- I. Hutchinson, M. F. Stevens and A. D. Westwell, Tetrahedron Lett., 2000, 41, 425–428 CrossRef CAS.
- S. Pal, G. Patra and S. Bhunia, Synth. Commun., 2009, 39, 1196–1203 CrossRef CAS.
- D. Ma, S. Xie, P. Xue, X. Zhang, J. Dong and Y. Jiang, Angew. Chem., Int. Ed., 2009, 48, 4222–4225 CrossRef CAS PubMed.
- T. Mase and T. Itoh, Pure Appl. Chem., 2008, 80, 707–715 CrossRef CAS.
- C. L. Li, X. G. Zhang, R. Y. Tang, P. Zhong and J. H. Li, J. Org. Chem., 2010, 75, 7037–7040 CrossRef CAS PubMed.
- S. Murru, H. Ghosh, S. K. Sahoo and B. K. Patel, Org. Lett., 2009, 11, 4254–4257 CrossRef CAS PubMed.
- S. Jayakumar, P. Singh and M. P. Mahajan, Tetrahedron, 2004, 60, 4315–4324 CrossRef CAS PubMed.
- D.-Q. Shi, S.-F. Rong and G.-L. Dou, Synth. Commun., 2010, 40, 2302–2310 CrossRef CAS.
- V. Bénéteau, T. Besson and C. W. Rees, Synth. Commun., 1997, 27, 2275–2280 CrossRef.
- P. S. Yadav, D. Devprakash and G. P. Senthilkumar, Int. J. Pharm. Sci. Drug Res., 2011, 3, 1–7 CAS.
- C. S. Schneider and J. Mierau, J. Med. Chem., 1987, 30, 494–498 CrossRef CAS.
- R. Deshmukh, A. S. Thakur, A. K. Jha and R. Deshmukh, Int. J. Res. Pharm. Chem., 2011, 1, 329–333 CAS.
- D. Munirajasekhar, M. Himaja and V. Mali sunil, Int. Res. J. Pharm., 2011, 2, 114–117 CAS.
- J. K. Malik, F. Manvi, B. Nanjwade and S. Singh, Drug Invent. Today, 2009, 1, 32–34 CAS.
- T. R. Manju thej, K. C. Chaluvaraju, M. S. Niranjan, M. Zaranappa and M. Krishnappa, Int. J. Pharm., 2012, 1, 75–80 Search PubMed.
- G. Alang, R. Kaur, A. Singh, P. Budhlakoti, A. Singh and R. Sanwal, Int. J. Pharm. Biol. Arch., 2010, 1, 56–61 Search PubMed.
- A. W. Chow, S. P. Bitler, P. E. Penwell, D. J. Osborne and J. F. Wolfe, Macromolecules, 1989, 22, 3514–3520 CrossRef CAS.
- D.-F. Shi, T. D. Bradshaw, S. Wrigley, C. J. McCall, P. Lelieveld, I. Fichtner and M. F. Stevens, J. Med. Chem., 1996, 39, 3375–3384 CrossRef CAS PubMed.
- S.-T. Huang, I.-J. Hsei and C. Chen, Bioorg. Med. Chem., 2006, 14, 6106–6119 CrossRef CAS PubMed.
- M. A. Lyon, S. Lawrence, D. J. Williams and Y. A. Jackson, J. Chem. Soc., Perkin Trans. 1, 1999, 437–442 RSC.
- R. Wang, D. Liu, K. Xu and J. Li, J. Photochem. Photobiol., A., 2009, 205, 61–69 CrossRef CAS PubMed.
- M. Khoobi, S. Emami, G. Dehghan, A. Foroumadi, A. Ramazani and A. Shafiee, Arch. Pharm., 2011, 344, 588–594 CrossRef CAS PubMed.
- M. Khoobi, A. Ramazani, A. Foroumadi, H. Hamadi, Z. Hojjati and A. Shafiee, J. Iran. Chem. Soc., 2011, 8, 1036–1042 CrossRef CAS.
- P. P. Deohate, Chem. Sci. Trans., 2013, 2, 473–478 CrossRef.
- G. Greco, L. Panzella, A. Napolitano and M. d’Ischia, Tetrahedron Lett., 2009, 50, 3095–3097 CrossRef CAS PubMed.
- D. C. Lynch, J. R. Miller and T. D. Spawn, Synth. Commun., 1997, 27, 897–905 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |