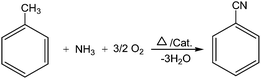Characterization and catalytic properties of molybdenum oxide catalysts supported on ZrO2–γ-Al2O3 for ammoxidation of toluene
Abbas Teimouri*a,
Bahareh Najaria,
Alireza Najafi Chermahinib,
Hossein Salavatia and
Mahmoud Fazel-Najafabadic
aChemistry Department, Payame Noor University, 81395-671, 19395-3697, Tehran, Isfahan, I. R. of Iran. E-mail: a_teimouri@pnu.ac.ir; a_teimoory@yahoo.com; Fax: +98 31 33521802; Tel: +98 31 33521804
bDepartment of Chemistry, Isfahan University of Technology, Isfahan, 841543111, Iran
cMechanical Engineering Department, Payame Noor University, 19395-3697, Tehran, I. R. of Iran
First published on 4th August 2014
Abstract
Molybdenum oxide catalysts with MoO3 loadings ranging from 6.6 to 25 wt% supported on ZrO2–γ-Al2O3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 wt%) mixed oxide were prepared by a wet impregnation method. The catalytic behavior of catalysts in the toluene ammoxidation reaction was investigated in a lab-scale tube reactor at 400 °C. The catalytic performance of MoO3/ZrO2–γ-Al2O3 was dependent on the catalyst compositions and reaction temperature. MoO3 (20.0 wt%) ZrO2–γ-Al2O3 exhibited a good toluene oxidation; over this catalyst, the selectivity to benzonitrile reached 67.0% with a toluene conversion of 68.5% at 400 °C, while the selectivity to benzaldhyde was 24.4% with a toluene conversion of 68.5% at 400 °C. The catalysts were characterized by various techniques, such as N2 sorption, FTIR, SEM and XRD.
1 wt%) mixed oxide were prepared by a wet impregnation method. The catalytic behavior of catalysts in the toluene ammoxidation reaction was investigated in a lab-scale tube reactor at 400 °C. The catalytic performance of MoO3/ZrO2–γ-Al2O3 was dependent on the catalyst compositions and reaction temperature. MoO3 (20.0 wt%) ZrO2–γ-Al2O3 exhibited a good toluene oxidation; over this catalyst, the selectivity to benzonitrile reached 67.0% with a toluene conversion of 68.5% at 400 °C, while the selectivity to benzaldhyde was 24.4% with a toluene conversion of 68.5% at 400 °C. The catalysts were characterized by various techniques, such as N2 sorption, FTIR, SEM and XRD.
Introduction
Ammoxidation of alkyl aromatics such as toluene to their corresponding nitriles has been the subject of numerous studies in recent times, because the nitriles are very useful organic intermediates to prepare a good number of industrially important chemicals.1,2 The ammoxidation reaction generally refers to the one-step formation of nitrile compounds in a single step by the oxidation of simple olefins, aromatics and heteroaromatics in the presence of oxygen and ammonia in the gas phase.3–5 Supported molybdenum oxide catalysts are well known and widely investigated as they represent an important group of catalysts for the heterogeneous oxidation and ammoxidation of hydrocarbons.6–17Because pure MoO3 is relatively volatile, molybdena is almost always used in the presence of a second oxide, on an oxide support such as Al2O3, TiO2, ZrO2, SiO2 and MgO.6,11,12,14,18–23
The desirable inherent properties of alumina and zirconia supports can be explored by combination of both supports in a mixed oxide. The ZrO2–γ-Al2O3 supported catalysts have been found to show better catalytic properties than catalysts supported on pure oxides.24,25 The combination of Al2O3 and ZrO2 provides greater mechanical strength, resulting in improved resistance to attrition.26,27 In recent times, ZrO2–γ-Al2O3 based materials have been employed as catalysts in various catalytic applications.28,29 The advantages of Al2O3–ZrO2 as a catalyst support include moderate surface area, higher thermal stability and medium acidity. The ammoxidation of toluene25,31–53 and other alkyl aromatics30,52–63 over various supported metal oxide catalysts has been extensively studied. Iron,64–66 MoO3/MgF2,67 MoO3/ZrO2,68,69 V2O5/ZrO2–γ-Al2O3,70,71 V2O5/γ-Al2O3,72 Mo–V–P/γ-Al2O3,73 vanadium-containing catalysts,74,75 Fe2O3-based catalysts,76 ZrO2–γ-Al2O3,77 and SiO2-supported molybdate catalyst,78 have also been used for the preparation of aromatic nitriles.
In the present study, we report the synthesis of benzonitrile by the vapor phase ammoxidation over highly dispersed molybdena catalysts supported on ZrO2–γ-Al2O3 mixed oxide, as shown in Scheme 1. The catalysts were characterized by X-ray diffraction (XRD), SEM, BET specific surface area and temperature programmed desorption of N2.
Experimental section
Materials and instruments
Toluene and other agents were purchased from Merck and Aldrich and used without further purification. Products were characterized by spectroscopy data (FTIR, 1H NMR and 13C NMR spectra). NMR spectra were recorded on a Bruker 400 Ultra shield NMR and DMSO-d6 was used as the solvent. Mass Spectra were recorded on a Shimadzu Gas Chromatograph Mass Spectrometer GCMS-QP5050A/Q P5000 apparatus.The samples were analyzed by X-ray diffraction (XRD) using Philips X'PERT MPD X-ray diffractometer (XRD) with Cu Kα° (1.5405 Å). Date sets were collected over the range of 5–90° with a step size of 0.02° and a count rate of 3.0° min−1. The structural morphology of the samples was evaluated using scanning electron microscope (SEM, JEOL, JSM-6300, Tokyo, Japan). A JASCO FT/IR-680 PLUS spectrometer was applied to record IR spectra using KBr pellets. The BET specific surface areas and BJH pore size distribution of the samples were determined by adsorption–desorption of nitrogen at liquid nitrogen temperature using a Series BEL SORP 18.
Catalyst preparation
The MoO3/ZrO2 and MoO3/γ-Al2O3 catalysts were prepared by impregnation of γ-Al2O3 or ZrO2 with a 2 M oxalic solution of ammonium heptamolybdate. The mixture was left in an open vessel with stirring at 60 °C for 24 h to evaporate the excess water. The precursor was dried at 100 °C for 12 h and calcined at 500 °C for 6 h before use.A series of MoO3/ZrO2/γ-Al2O3 catalysts with MoO3 loadings in the range of 6.6–25.0 wt% were prepared by wet impregnation method. To impregnate MoO3, the calculated amount of ammonium heptamolybdate was dissolved in 30–40 ml doubly distilled water and reflux at 85–90 °C for 5 h. Then, a few drops of dilute NH4OH were added to make the solution clear and keep the pH constant (pH = 8). After impregnation, the reaction mixture was added to a 50 ml Pyrex flask. The mixture was irradiated in the water bath of the ultrasonic at 20 kHz for 1 h within the temperature range of 25–30 °C. Then the catalysts were dried at 85–90 °C for about 4 h and calcined at 500 °C for 6 h before use.
Ammoxidation of toluene
A stainless steel cylindrical micro reactor (i.d. 4.8 cm; a reactor length of 8.55 cm; volume 150 cm3), was charged with toluene (3 ml), 20 mg catalyst and a magnetic stirring bar. The autoclave was purged and filled with NH3 until the pressure reached 0.75 MPa. Then O2 was introduced until the total pressure reached to 1.25 MPa. The reaction mixture was stirred at a controlled temperature (400 °C for 2 h). After the reaction, the mixture was filtered. The filtrate was analyzed by GC-MS and GC using benzonitrile as an internal standard. For recycling tests, the catalyst was filtered after the reaction, washed with acetone three times and then with doubly distilled water several times. Then, it was dried at 110 °C, calcined at 400 °C for 4 h, and then used for the next run.Conversion and selectivity were defined as follows
| • C (mol%) = (mol toluene reacted/mol toluene in the feed) × 100 |
| • Si (mol%) = (mol i formed/mol toluene reacted) × 100 |
Results and discussion
XRD analysis
X-ray diffraction (XRD) patterns of the catalysts were obtained using Cu Kα radiation (λ = 1.5405 Å). Crystallite size of the crystalline phase was determined from the peak of maximum intensity by using Scherrer formula,79 with a shape factor (K) of 0.9, which can be described as: crystallite size = Kλ/W![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ, where W = Wb − Ws and Wb is the broadened profile width of experimental sample and Ws is the standard profile width of reference silicon sample.
θ, where W = Wb − Ws and Wb is the broadened profile width of experimental sample and Ws is the standard profile width of reference silicon sample.
Fig. 1A shows the XRD patterns of MoO3, MoO3/ZrO2 and MoO3/γ-Al2O3 samples. The peaks presented at 2θ = 20–30° are attributed to the pure MoO3, Fig. 1A(a). The XRD pattern of MoO3/ZrO2 showed peaks at 2θ = 30, 50 and 60, which were obviously the characteristics of the tetragonal ZrO2. The X-ray diffraction pattern of MoO3/γ-Al2O3 exhibit broad peaks at 2θ = 45 and 66°, which were attributed to γ-Al2O3, Fig. 1A(c).
X-ray diffraction patterns of the catalysts with different loadings of molybdena catalysts supported on Al2O3–ZrO2 and calcined at 450 °C are shown in Fig. 1B. The catalysts showed characteristic peaks at 2θ = 32.8, 37.3, 45.9, 62.3 and 66.09° that were related to the support γ-alumina. The sharp diffraction lines at 2θ = 30.4, 51.0 and 60.2° corresponded to the tetragonal ZrO2 phase. Loading of molybdenum species led to the appearance of new peaks at 2θ = 12.8, 23.9, 22.2, 25.7, 28.5, 34.2 and 39.4°.
FT-IR analysis
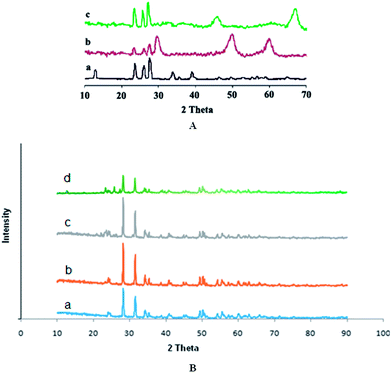
The FTIR spectrum for MoO3 is presented for the range, 350–4000 cm−1 in Fig. 2A(a). The bands at 991, 870, and 491 cm−1, were assigned to the Mo![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching mode, the Mo–O–Mo stretching mode, and MoO3 vibration mode, respectively. For MoO3/ZrO2 catalyst in Fig. 2A(b), FTIR band at 989 cm−1 was due to the Mo
O stretching mode, the Mo–O–Mo stretching mode, and MoO3 vibration mode, respectively. For MoO3/ZrO2 catalyst in Fig. 2A(b), FTIR band at 989 cm−1 was due to the Mo![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching mode of the molybdenum oxide complex bonded to the ZrO2 surface. Molybdenum oxides species was stabilized through multiple Mo–O–Zr bonds between each molybdenum oxide species and the zirconia surface. The FTIR spectrum for MoO3/γ-Al2O3 catalyst is shown in Fig. 2A(c). The band at 899 cm−1 characterized the stretching mode of the Mo
O stretching mode of the molybdenum oxide complex bonded to the ZrO2 surface. Molybdenum oxides species was stabilized through multiple Mo–O–Zr bonds between each molybdenum oxide species and the zirconia surface. The FTIR spectrum for MoO3/γ-Al2O3 catalyst is shown in Fig. 2A(c). The band at 899 cm−1 characterized the stretching mode of the Mo![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond in surface-bound Mo species. These species could be either isolated tetrahedral or octahedral polymolybdate species.
O bond in surface-bound Mo species. These species could be either isolated tetrahedral or octahedral polymolybdate species.
FT-IR spectra of the supports and molybdenum catalysts are shown in Fig. 2B. A broad band in the range of 350–4000 cm−1 appeared for all catalysts related to the MoO3 species (including the vibrations of Mo–O, bridging oxygen corresponding to Mo–O–Mo). The bands at 878, 734 and 497 cm−1 corresponding to the polymolybdates species. The spectrum in Fig. 2B exhibited bands at 3450–3760 cm−1, typical of the ν OH bands of alumina hydroxyls. The band at 3764 cm−1 was assigned to basic hydroxyl groups bound to a single tetrahedrally coordinated aluminum atom, while the band at 3642 cm−1 was due to bridged OH groups shared by an octahedrally and tetrahedrally coordinated aluminum cation. The appearance of the band around 1050 cm−1 was typical for γ-alumina due to Al–O vibration mode. On the other hand, bands that appeared at 1632 and 2350 cm−1 were related to physisorbed water and OH group free from the interaction of H bonding respectively. There was a strong absorption band at 417 cm−1 which could be attributed to the tetragonal zirconia. At higher MoO3 loading, the bands due to microcrystallites MoO3 appeared at 518, 734, and 878 cm−1. The increase in the intensity of this band with MoO3 loading indicated the growth of polymolybdate species. These bands were associated with Mo–O–Al and Mo![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond vibration in aluminum molybdate and crystalline MoO3 phases, respectively.
O bond vibration in aluminum molybdate and crystalline MoO3 phases, respectively.
SEM analysis
Fig. 3 shows SEM micrographs of catalysts obtained from MoO3 loadings ranging from 6.6 to 25.0 wt%. Alumina displayed an irregular texture and accumulated aggregates with a variety of particles size. This indicated that the introduction of ZrO2 into Al2O3 largely changed the morphology of the support composites. It can be seen from Fig. 3 that the particles seemed to aggregate to form microspheres. SEM shows a regular texture with small, uniform and dispersed particles. | ||
| Fig. 3 SEM images of the molybdate supported catalysts (a) 6.6% wtMoO3/ZrO2–γ-Al2O3, (b) 12.5 wt% MoO3/ZrO2–γ-Al2O3, (c) 20.0 wt% MoO3/ZrO2–γ-Al2O3, (d) 25.0 wt% MoO3/ZrO2–γ-Al2O3. | ||
BET analysis
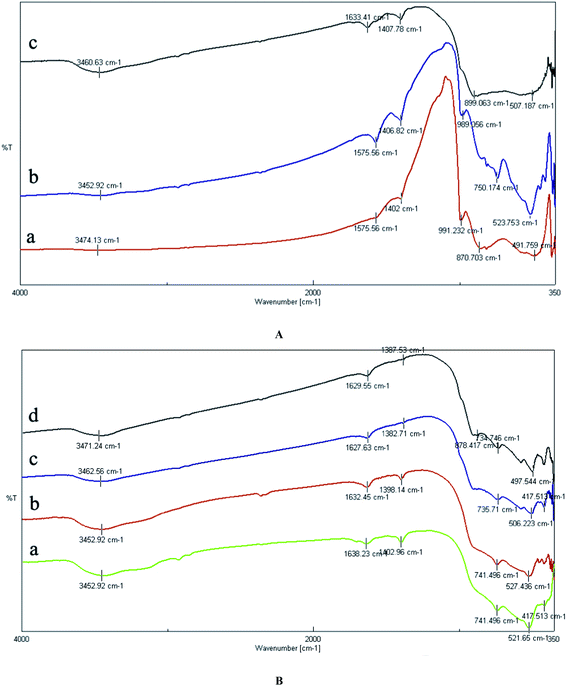
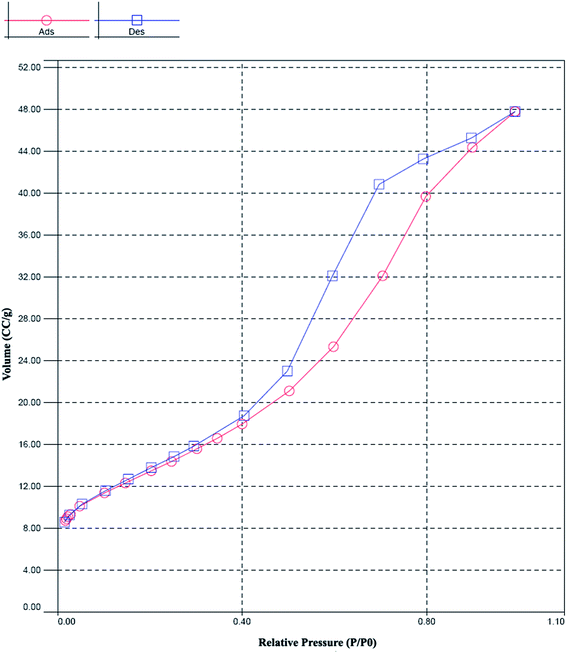
Fig. 4 shows the N2 adsorption–desorption isotherms. Surface area was calculated by applying the BET equation to the isotherm.27 The samples were degassed under vacuum at 120 °C for 4 h, prior to adsorption measurement, to evacuate the physisorbed moisture.The effects of catalyst composition and reaction temperature on the toluene conversion and product distribution for toluene oxidation over MoO3/ZrO2–γ-Al2O3 are illustrated in Table 1. As shown, the toluene conversion reached a maximum over the catalyst with the MoO3 loading of 20.0 wt% under each reaction temperature, while the selectivity to the main products fluctuated with the increase of MoO3 loading.
| MoO3 loading | T (°C) | Toluene conversion (%) | Product selectivity (%) | ||
|---|---|---|---|---|---|
| Benzonitrile | Benzene | Benzaldehyde | |||
| 6.6% | 200 | 28.6 | 40.7 | ≤1 | 20.5 |
| 300 | 44.5 | 46.2 | 1.3 | 23.7 | |
| 400 | 44.2 | 63.5 | 2 | 24.4 | |
| 12.5% | 200 | 32.5 | 46.3 | ≤1 | 20.5 |
| 300 | 48.4 | 50.4 | 1.3 | 23.7 | |
| 400 | 56.6 | 66.4 | 2 | 24.4 | |
| 20.0% | 200 | 44.2 | 40.3 | 1.3 | 23.7 |
| 300 | 64.6 | 58.6 | 2 | 24.4 | |
| 400 | 68.5 | 68 | ≤1 | 24.4 | |
| 25.0% | 200 | 30.3 | 35.9 | ≤1 | 20.5 |
| 300 | 43.7 | 47.5 | 1.3 | 23.7 | |
| 400 | 48.6 | 67.6 | 2 | 24.2 | |
The MoO3/ZrO2/γ-Al2O3 catalysts with different MoO3 contents were evaluated for the ammoxidation of toluene. The ammoxidation of toluene resulted in the formation of benzonitrile as the major product, while benzene and benzaldehyde were formed in very low amounts. The catalysts with the low loading of MoO3, up to 6.6 wt%, showed moderate activity and when the loading was increased to 20.0 wt%, a substantial increase in activity was observed. The catalyst with 20.0 wt% MoO3 exhibited the highest activity. With further increase in the active content to 25 wt%, the ammoxidation activity was decreased marginally. The low catalytic activity of 6.6–20.0 wt% MoO3/ZrO2/γ-Al2O3 catalysts might be because of the less availability of active MoO3 compound. With the increase of the reaction temperature, the toluene conversion and the selectivity to benzonitrile and benzaldehyde were increased, while the selectivity to benzene was decreased. Over this catalyst, the selectivity to benzonitrile reached to 67.0% with the toluene conversion of 68.5% at 400 °C, while the selectivity to benzonitrile was 58.6% with the toluene conversion of 64.6% at 300 °C. A significant drop in surface area occurred when molybdena loading was increased from 6.6 to 20.0 wt%. Such a decrease might be due to either the blockage of some pores of ZrO2/γ-Al2O3 by mixed oxides formed from the decomposition of molybdate or the solid-state reaction between the supporting oxides and the dispersed active oxides.80–82 Table 2 shows the BET surface area values of the catalysts. The surface area and pore volume of the MoO3/ZrO2–γ-Al catalysts were in the range of 48–116 m2 g−1 and 0.50–0.62 cm3 g−1, respectively. A gradual decrease in surface area was observed for the catalysts with an increase in the loading of molybdena supported on ZrO2/γ-Al2O3, but an increase in the average pore diameters. This phenomenon might be due to two reasons. One refers to MoO3 particles deposited in the pores of ZrO2–γ-Al2O3 and the blocked part of the small pores.
| Catalyst | BET surface area (m2g-1) | Surface density Mo/nm2 | Pore volume (cm3 g−1) | Average pore diameter (nm) |
|---|---|---|---|---|
| 6.6% MoO3/ZrO2–γ-Al2O3 | 116.26 | 2.37 | 0.78 | 6.22 |
| 12.5% MoO3/ZrO2–γ-Al2O3 | 78.13 | 6.69 | 0.74 | 6.23 |
| 20.0%MoO3/ZrO2–γ-Al2O3 | 66.32 | 12.61 | 0.68 | 6.25 |
| 25.0% MoO3/ZrO2–γ-Al2O3 | 48.46 | 21.57 | 0.64 | 6.34 |
In addition, the Mo surface density values are measured and data are presented in Table 2. The surface density is defined as the number of Mo atoms per square nanometer BET surface area (Mo atoms nm−2). Surface areas decreased only slightly with increasing MoO3 loading; therefore, the Mo surface density increased almost linearly with increasing MoO3 concentration. In addition it has been noted that for Mo/Al2O3 system, formation of a polymolybdate monolayer on Al2O3 at surface densities of 4.8 Mo nm−2 occur.83 As you can see, the loading 6.6% MoO3 leads to formation of a molybdate monolayer on mixed oxide surface.
The other relates to the morphology of composite supports changed from big blocks into small particles (observed from SEM image), thereby forming more inter pores between the particles. All samples were mesoporous, with N2 adsorption–desorption isotherms of type IV according to the IUPAC classification. Such isotherms are shown in Fig. 4, which shows the case of a bare support taken as a representative example for 20.0 wt% MoO3/ZrO2–γ-Al2O3 catalyst.
One of the most important advantages of heterogeneous catalysis over the homogeneous counterpart is the possibility of reusing the catalyst by simple filtration, without loss of activity. The recovery and reusability of the catalyst were investigated in the product formation. After completion of the reaction, the catalyst was separated by filtration, washed first 3 times with 5 ml acetone and then with doubly distilled water several times, dried at 110 °C and calcined at 400 °C for 4 h. Then the recovered catalyst was used in the next run. The results of three consecutive runs showed that the catalyst could be reused several times without any significant loss of its activity (see Fig. 5).
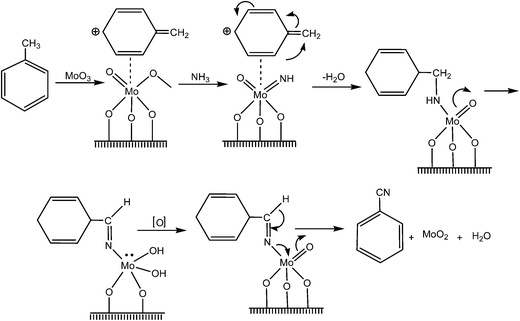
It is generally accepted that the reaction proceeds by the adsorption of toluene on the catalyst surface through the formation of a π-complex with a Lewis site of the catalyst; furthermore, we should consider H abstraction of a benzylic H-atom to form a methylene-like species with parallel formation of water, partial oxidation, N-insertion and subsequent rearrangements of the chemisorbed activated surface species, which was converted to an adsorbed imine, and desorption of the so formed benzonitrile, which was followed by oxidative reconstruction of the catalyst surface (Fig. 6). A similar mechanism has been proposed for this reaction.84
This was also reflected in the catalytic activity of these catalysts. Conversion of toluene to benzonitrile was increased continuously with molybdena loading up to 20.0 wt%. It indicated that the moderate and weak acidic sites played an important role in the ammoxidation of toluene.
The results of ammoxidation of toluene on various MoO3/ZrO2–γ-Al2O3 catalysts at 400 °C are plotted in Fig. 7. The conversion and selectivity were increased with an increase in MoO3 loading up to 20.0 wt% and beyond this loading, the activity was decreased slightly due to the formation of MoO3crystallites on the surface of ZrO2–γ-Al2O3 support. The increase in the ammoxidation activity of the catalysts might be attributed to the increase in the number of sites on the active molybdena phase, which could be increased with the increase in molybdena content on the surface of the support. The surface properties and catalytic activity results of 20.0 wt% MoO3 supported onmixed oxide alumina–zirconia catalysts have been compared in Table 2. It clearly shows that molybdena was well dispersed on MoO3/ZrO2–γ-Al2O3 support, with more acidic sites per m2 surface of the support.
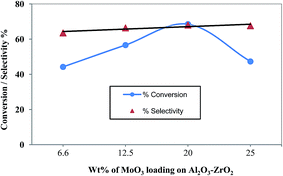 | ||
| Fig. 7 Ammoxidation of toluene over various MoO3/ZrO2–Al2O3 catalysts (reaction temperature of 400 °C). | ||
Thus, it can be inferred that 20.0 wt% MoO3/ZrO2–γ-Al2O3 catalyst can be more active in ammoxidation reaction compared to a time when it is supported on alumina–zirconia catalysts.
Benzonitrile characterization
FTIR (KBr, cm−1): 3116, 3064, 2256, 1662, 1098, 625 cm−1; 1H NMR (400 MHz, DMSO-d6): δ 7.383–7.412 (m, 2H), 7.518–7.561 (m, 3H); 13C NMR (100 MHz, DMSO-d6): δ 112.30 (1C), 118.82 (1C), 129.16 (2C), 132.05 (2C), 132.82 (1C); ESI MS (m/z): 103.08 (M+).Conclusions
The catalyst MoO3/ZrO2–γ-Al2O3 exhibited excellent catalytic performance in toluene ammoxidation with benzonitrile as the main product. The Al2O3–ZrO2 binary oxide was found to be an interesting support to investigate the dispersion of molybdenum oxide and catalytic properties. The catalytic performance of MoO3/ZrO2–γ-Al2O3 was dependent on the catalyst compositions and reaction temperature. Increasing the MoO3 loading from 6.6 to 25.0 wt% enhanced the activity of the catalyst. Above 20.0 wt%, however, it led to inactivity and performance failure of the catalyst. Over this catalyst, the selectivity to benzonitrile reached 67.0% with the toluene conversion of 68.5% at 400 °C, while the selectivity to benzonitrile was 58.6% with the toluene conversion of 64.6% at 300 °C.Conflict of interest
The authors declare no competing financial interest.Acknowledgements
The authors would like to thank the Iranian National Science Foundation (INSF) for financial support of this work. Supports from Isfahan research council at Payame Noor University and the help from Isfahan University of technology are gratefully acknowledged.Notes and references
- V. M. Bondareva, T. V. Andrushkevich, E. A. Paukshtis, N. A. Paukshtis, A. A. Budneva and V. N. Parmon, J. Mol. Catal. A: Chem., 2007, 269, 240–245 CrossRef CAS PubMed.
- R. G. Rizayev, E. A. Mamedov, V. P. Vislovskii and V. E. Sheinin, Appl. Catal., A, 1992, 83, 103–140 CrossRef.
- R. K. Grasselli and M. A. Tenhover in Handbook of Heterogeneous Catal, ed. G. Ertl, H. Knçzinger, F. Schth and J. Weitkamp, Wiley-VCH, Weinheim, 2008, pp. 3489–3517 Search PubMed.
- A. Martin and B. Lcke, Catal. Today, 2000, 57, 61–70 CrossRef CAS.
- R. K. Grasselli, Catal. Today, 1999, 49, 141–153 CrossRef CAS.
- G. Tsilomelekis, A. Christodoulakis and S. Boghosian, Catal. Today, 2007, 127, 139–147 CrossRef CAS PubMed.
- G. C. Behera, K. Parida, N. F. Dummer, G. Whiting, N. Sahu, A. F. Carley, M. Conte, G. J. Hutchings and J. K. Bartley, Catal. Sci. Technol., 2013, 3, 1558–1564 CAS.
- S. T. Oyama, R. Radhakrishnan, M. Seman, J. N. Kondo, K. Domen and K. Asakura, J. Phys. Chem. B, 2003, 107, 1845–1852 CrossRef CAS.
- R. Nie, J. Shi, S. Xia, L. Shen, P. Chen, Z. Hou and F.-S. Xiao, J. Mater. Chem., 2012, 22, 18115–18118 RSC.
- E. V. Ishchenko, T. V. Andrushkevich, G. Ya. Popova, T. Yu. Kardash, A. V. Ishchenko, L. S. Dovlitova and Yu. A. Chesalov, Appl. Catal., A, 2014, 476, 91–102 CrossRef CAS PubMed.
- X. B. Ma, J. L. Gong, S. P. Wang, N. Gao, D. L. Wang, X. Yang and F. He, Catal. Commun., 2004, 5, 101–106 CrossRef CAS PubMed.
- S. Zhang, Q. Zhong, W. Zhao and Y. Li, Chem. Eng. J., 2014, 253, 207–216 CrossRef CAS PubMed.
- G. M. Dharand, B. N. Srinivas, M. S. Ranaand, M. Kumarand and S. K. Maity, Catal. Today, 2003, 86, 45–60 CrossRef.
- K. V. R. Chary, K. R. Reddy, G. Kishan, J. W. Niemantsverdriet and G. Mestl, J. Catal., 2004, 226, 283–291 CrossRef CAS PubMed.
- Catal. from A to Z: a Concise Encyclopedia, ed. B. Cornils, W. A. Herrmann, M. Muhler and C.H. Wong, Wiley-VCH, Weinheim,Germany, 3rd edn, 2007 Search PubMed.
- M. L. Pacheco, J. Soler, A. Dejoz, J. M. Lopez Nieto, J. Hergiudo, M. Menendez and J. Santamaria, Catal. Today, 2000, 61, 101–107 CrossRef CAS.
- J. E. Miller, N. B. Jackson, L. Evans, A. G. Sault and M. M. Gonzates, Catal. Lett., 1999, 12, 147–152 CrossRef.
- R. Radhakrishnan, C. Reed, S. T. Oyama, M. Seman, J. N. Kondo, K. Domen, Y. Ohminami and K. Asakura, J. Phys. Chem. B, 2001, 105, 8519–8530 CrossRef CAS.
- K. Y. S. Ng and E. Gulari, J. Catal., 1985, 92, 340–354 CrossRef CAS.
- Y. S. Jin, A. Auroux and J. C. Vedrine, J. Chem. Soc., Faraday Trans., 1989, 83, 4179–4191 RSC.
- H. Miyata, S. Toukuda, T. Ono and F. Hatayama, J. Chem. Soc., Faraday Trans., 1990, 86, 2291 RSC.
- T. Ono, H. Miyata and Y. Kubokaw, J. Chem. Soc., Faraday Trans., 1987, 83, 1761–1770 RSC.
- H. Miyata, S. Toukuda, T. Ono, T. Othno and F. Hatayama, J. Chem. Soc., Faraday Trans., 1990, 86, 3679–3682 RSC.
- M. Sanati and A. Andersson, Appl. Catal., A, 1993, 106, 51–72 CrossRef CAS.
- Y. Murakami, M. Niwa, T. Hattori, S. Osawa, I. Igushi and H. Ando, J. Catal., 1977, 49, 83–91 CrossRef CAS.
- A. Sahibed-Dine, B. Bouanis, K. Nohair and M. Bensitel, Ceram. Int., 2002, 28, 159–164 CrossRef.
- S. C. Farmer and A. Sayir, Eng. Fract. Mech., 2002, 69, 1015–1024 CrossRef.
- C. Larese, J. M. Campos-Martin, J. J. Calvino, G. Blanco, J. L. G. Fierro and Z. C. Kang, J. Catal., 2002, 208, 467–478 CrossRef CAS.
- S. Castillo, M. Moran-Pineda and R. Gomez, Catal. Commun., 2001, 2, 295–300 CrossRef CAS.
- R. G. Rizayev, E. A. Mamedov, V. P. Vislovskii and V. E. Sheinin, Appl. Catal., A, 1992, 83, 103–140 CrossRef.
- M. Niwa, H. Ando and Y. Murakami, J. Catal., 1977, 49, 92–96 CrossRef CAS.
- Y. Murakami, H. Ando and M. Niwa, J. Catal., 1981, 67, 472–474 CrossRef CAS.
- M. Niwa, M. Sago, H. Ando and Y. Murakami, J. Catal., 1981, 69, 69–76 CrossRef CAS.
- M. Niwa and Y. Murakami, J. Catal., 1982, 76, 9–16 CrossRef CAS.
- P. Cavalli, F. Cavani, I. Manenti and F. Trifiro, Ind. Eng. Chem. Res., 1987, 26, 639–647 CrossRef CAS.
- P. Cavalli, F. Cavani, I. Manenti, F. Trifiro and M. El-Sawi, Ind. Eng. Chem. Res., 1987, 26, 804–810 CrossRef CAS.
- G. Busca, F. Cavani and F. Trifiro, J. Catal., 1987, 106, 471–482 CrossRef CAS.
- J. C. Otamiri and A. Andersson, Catal. Today, 1988, 3, 211–222 CrossRef CAS.
- J. C. Otamiri and A. Andersson, Catal. Today, 1988, 3, 223–234 CrossRef CAS.
- A. Andersson and S. Hansen, J. Catal., 1988, 114, 332–346 CrossRef CAS.
- M. Sanati and A. Andersson, Ind. Eng. Chem. Res., 1991, 30, 312–320 CrossRef CAS.
- M. Sanati and A. Andersson, Ind. Eng. Chem. Res., 1991, 30, 320–326 CrossRef CAS.
- M. Sanati, L. R. Wallenberg, A. Andersson, S. Jansen and Y. Tu, J. Catal., 1991, 32, 128–144 CrossRef.
- S. Jansen, Y. Tu, M. J. Palmieri, M. Sanati and A. Andersson, J. Catal., 1992, 138, 79–89 CrossRef CAS.
- M.-D. Lee, W.-S. Chen and H.-P. Chiang, Appl. Catal., A, 1993, 101, 269–281 CrossRef CAS.
- M. Sanati, A. Andersson, L. R. Wallenberg and B. Rebenstorf, Appl. Catal., A, 1993, 106, 51–72 CrossRef CAS.
- A. Andersson, S. L. T. Andersson, G. Centi, R. K. Grasselli, M. Sanati and F. Trifiro, Appl. Catal., A, 1994, 113, 43–57 CrossRef CAS.
- S. J. Kulkarni, R. Ramachandra Rao, M. Subrahmanyam, A. V. Rama Rao, A. Sarkani and L. Guczi, Appl. Catal., A, 1996, 139, 59–74 CrossRef CAS.
- K. V. R. Chary, K. R. Reddy, T. Bhaskar and G. V. Sagar, Green Chem., 2002, 4, 206–209 RSC.
- C. P. Kumar, K. R. Reddy, V. V. Rao and K. V. R. Chary, Green Chem., 2002, 4, 513–516 RSC.
- K. Smeykal, K.-K. Moll, E. Heyner, K. Pelzing and U. Schattenholz, UK Patent 1124457, 1968.
- Handbook of Heterogeneous Catal, ed. R. K. Grasselli, G. Ertl, H. Knoezinger and J. Weitkamp, Wiley, Weinheim, 1997, vol. 5, pp. 2303–2379 Search PubMed.
- M. V. Landau, M. L. Kaliya and M. Herskowitz, Appl. Catal., A, 2001, 208, 21–34 CrossRef CAS.
- (a) K. V. Narayana, A. Martin, U. Bentrup, B. Lücke and J. Sans, Appl. Catal., A, 2004, 270, 57–64 CrossRef CAS PubMed; (b) N. Dropka, Q. Smejkal, V. N. Kalevaru and A. Martin, J. Catal., 2006, 240, 8–17 CrossRef CAS PubMed.
- N. Dropka, Q. Smejkal, V. N. Kalevaru and A. Martin, Appl. Catal., A, 2008, 349, 125–132 CrossRef CAS PubMed.
- A. Martin, V. N. Kalevaru and Q. Smejkal, Catal. Today, 2010, 157, 275–279 CrossRef CAS PubMed.
- A. B. Azimov, V. P. Vislovskii, E. A. Mamedov and R. G. Rizayev, J. Catal., 1991, 127, 354–360 CrossRef CAS.
- G. Centi, Appl. Catal., A, 1996, 147, 267–298 CrossRef CAS.
- R. K. Grasselli, Catal. Today, 1999, 49, 141–153 CrossRef CAS.
- A. Martin and B. Lücke, Catal. Today, 2000, 57, 61–70 CrossRef CAS.
- A. Martin, N. V. Kalevaru, B. Lucke and J. Sans, Green Chem., 2002, 4, 481–485 RSC.
- A. Martin, U. Bentrup and G.-U. Wolf, Appl. Catal., A, 2002, 227, 131–142 CrossRef CAS.
- A. Martin, V. N. Kalevaru and B. Lucke, Catal. Today, 2003, 78, 311–317 CrossRef CAS.
- P. Nagaraju, N. Lingaiah, M. Balaraju and P. S. Sai Prasad, Appl. Catal., A, 2008, 339, 99–107 CrossRef CAS PubMed.
- P. Nagaraju, Ch. Srilakshmi, N. Pasha, N. Lingaiah, I. Suryanarayana and P. S. Sai Prasad, Catal. Today, 2008, 131, 393–401 CrossRef CAS PubMed.
- M. D. Allen, G. J. Hutchings and M. Bowker, Appl. Catal., A, 2001, 217, 33–39 CrossRef CAS.
- J. Haber and M. Wojciechowska, Catal. Lett., 1991, 10, 271–278 CrossRef CAS.
- T. Bhaskar, K. R. Reddy, C. P. Kumar, M. R. V. S. Murthy and K. V. R. Chary, Appl. Catal., A, 2001, 211, 189–201 CrossRef CAS.
- K. V. R. Chary, K. R. Reddy, G. Kishan, J. W. Niemantsverdriet and G. Mestl, J. Catal., 2004, 226, 283–291 CrossRef CAS PubMed.
- K. V. R. Chary, C. P. Kumar, P. V. R. Rao and V. V. Rao, Catal. Commun., 2004, 5, 479–484 CrossRef CAS PubMed.
- Y. Zhao, Z. Qin, G. Wang, M. Dong, L. Huang, H. Wua, W. Fanand and J. Wang, Fuel, 2013, 104, 22–27 CrossRef CAS PubMed.
- Y. Jeon, S. W. Row, A. Dorjgotov, S. D. Lee, K. Oh and Y. G. Shul, Korean J. Chem. Eng., 2013, 30(5), 1–5 Search PubMed.
- B. Hari Babu, G. Parameswaram, A. Sri Hari Kumar, P. S. Sai Prasad and N. Lingaiah, Appl. Catal., A, 2012, 445–446 Search PubMed.
- A. Martin and B. Lucke, Catal. Today, 2000, 57, 61–70 CrossRef CAS.
- A. Martin, Ur. Bentrup and G. U. Wolf, Appl. Catal., A, 2002, 227, 131–142 CrossRef CAS.
- E. Rombi, I. Ferino, R. Monaci, C. Picciau, V. Solinas and R. Buzzoni, Appl. Catal., A, 2004, 266, 73–79 CrossRef CAS PubMed.
- K. V. R. Chary, C. P. Kumara, D. Naresh, T. Bhaskar and Y. Sakata, J. Mol. Catal. A: Chem., 2006, 243, 149–157 CrossRef CAS PubMed.
- M. A. de Boer, A. J. A. Van Dillen, D. C. A. Koningsberger, J. W. A. Geus, M. A. A. Vuurman and I. E. A. Wachs, Catal. Lett., 1990, 11, 227–240 CrossRef.
- B. D. Cullity and S. R. Stock, Elements of X-ray Diffraction, Prentice Hall, Upper Saddle River, NJ, 3rd edn, 2001, p. 388 Search PubMed.
- K. V. R. A. Chary, C. P. A. Kumar, P. V. R. A. Rao and V. V. A. Rao, Catal. Commun., 2004, 5, 479–484 CrossRef CAS PubMed.
- Y. A. Murakami, M. A. Niwa, T. A. Hattori, S. i. A. Osawa, I. A. Igushi and H. A. Ando, J. Catal., 1977, 49, 83–91 CrossRef CAS.
- M. A. Sanati, A. A. Andersson, L. R. A. Wallenberg and B. A. Rebenstorf, Appl. Catal., A, 1993, 106, 51–72 CrossRef CAS.
- Y. Chen and L. F. Zheng, Catal. Lett., 1992, 12, 51–62 CrossRef CAS.
- J. A. Haber and M. A. Wojciechowska, J. Catal., 1988, 110, 23–36 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |