Reinforced magnetic epoxy nanocomposites with conductive polypyrrole nanocoating on nanomagnetite as a coupling agent†
Abstract
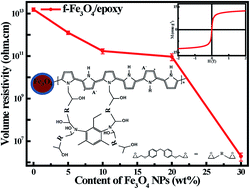
The new function of polypyrrole (PPy) to serve as a coupling agent has been demonstrated in preparing conductive epoxy resin nanocomposites with PPy coating on magnetite (f-Fe3O4) nanoparticles. The effects of magnetic nanofiller loading level on the rheological behavior, thermal stability, dynamic mechanical properties, mechanical properties, electrical conductivity, dielectric properties and magnetic properties were systematically studied. Compared with pure epoxy suspension, a reduced viscosity was observed in epoxy nanosuspensions with 5.0 wt% f-Fe3O4 nanoparticles, and the viscosity increased with further increasing f-Fe3O4 nanoparticle loading. Increased glass transition temperature (Tg) and enhanced mechanical tensile strength were observed in the cured solid epoxy polymer nanocomposites (PNCs) with f-Fe3O4 nanoparticles. The volume resistivity of the cured epoxy PNCs with 30.0 wt% f-Fe3O4 nanoparticles was decreased almost 7 orders of magnitude compared with the cured pure epoxy (1.6 × 1013 Ω cm). The cured epoxy PNCs exhibited good magnetic properties, and the surface functionality and epoxy matrix have little effect on the magnetic moment of the Fe3O4 nanoparticles. The role of PPy nanocoating on the nanocomposite formation mechanism was investigated by using the Fourier transform infrared spectroscopy (FT-IR) and thermogravimetric analysis (TGA) tests.

 Please wait while we load your content...
Please wait while we load your content...