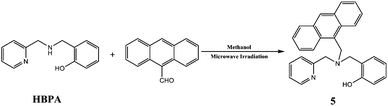
Efficient synthesis of new asymmetric tripodal ligands using microwave irradiation, and their crystal structures†
Zeli Yuanab,
Xiaoqing Yanga,
Lei Wanga,
Jiandong Huang*a and
Gang Wei*c
aCollege of Chemistry, Fuzhou University, Fuzhou 350108, P. R. China. E-mail: jdhuang@fzu.edu.cn; Fax: +86 591 22866227
bSchool of Pharmacy, Zunyi Medical University, Zunyi, Guizhou 563003, P. R. China. E-mail: zlyuan@zmc.edu.cn; Fax: +86 8528609343
cCSIRO Materials Science and Engineering, PO Box 218, Lindfield, NSW 2070, Australia. E-mail: gang.wei@csiro.au
First published on 15th August 2014
Abstract
An efficient method for the synthesis of asymmetric tripodal ligands from aryl aldehydes and (2-hydro-xybenzyl-2-pyri-dylmethyl)amine was developed. It has the advantages of short reaction times, high yields and a simple methodology. The title ligands were characterized using a combination of 1H NMR, 13C NMR, FT-IR and H RMS spectroscopies. The structures of some of the ligand were confirmed by single-crystal X-ray diffraction.
Nitrogen-rich tripodal ligands and their derivatives form stable complexes with a wide range of metal ions and their study continues to be of interest to researchers.1 Their versatile coordination modes lead to diverse roles in biological, catalytic and photoactive applications. These ligands have been investigated for use as catalysts in the sequential oxidation and asymmetric alkylation of alcohols by Ru3+ complexes.2 They have also been used as photochemotherapeutic agents in nuclear medicine, using Fe3+, Cu2+ or V4+ chelates;3 some of the ligands have been linked to suitable photosensitizer molecules, such as anthracenyl or ferrocene for activation by near FT-IR light, to enable greater tissue penetration.
Numerous complexes containing the basic N,N-bis-(2-pyridylmethyl)amine (DPA, Fig. 1) tripodal ligand has been included, and metal complexes containing this ligand have been synthesized, and metal complexes containing this ligand exhibit a wide variety of unusual physical and chemical properties.4 The denticity and donor types of these ligands determine the structures and reactivities of the corresponding metal complexes.5 Asymmetric substitution of the pyridyl units by a phenoxyl unit changes the reactive site,5a and the selective introduction of substituents on different units enables fine control of the steric and electronic properties of the ligand. However, previously reported asymmetric methods suffer from drawbacks such as long reaction times, low yields, high temperature, and large numbers of side products. The development of a new and effective methods for introducing asymmetry into tripodal ligands using inexpensive and environmentally friendly reagents is therefore needed. To the best of our knowledge, there have been few reports on the synthesis of asymmetric nitrogen- and oxygen-rich tripodal ligands.5a,6 Here, we focus on asymmetric tripodal ligands in which one ligand arm differs from the others, introducing the possibility of geometric isomers.
Organic reactions assisted by microwave irradiation have attracted considerable attention in recent years.7 Modern scientific microwave equipment can be used to control many reaction parameters such as temperature, pressure and reaction times accurately. Numerous microwave-assisted organic reactions have been performed,8 including Michael additions, acylation and alkylation reactions, condensations, enzymatic catalysis, rearrangements, oxidations and reductions, and regioselective cycloadditions.
The commonest of the method for the tripodal ligand synthesis is alkylation of DPA with 9-(chloromethyl)anthracene, using triethylamine as base.9 Usually, a large excess of one reagent and a long reaction time are needed to ensure high yields and to avoid undesired by-products. However, the preparation of 9-(chloromethyl)anthracene is inconvenient and expensive, and silica gel column chroma-tography using CH2Cl2 as eluant is used for product purification.
Secondary amines react readily with aromatic aldehydes to form enamines, which can then be reduced with appropriate reductant; this is an atom economic reaction,10 and therefore is in line with the philosophy of green chemistry. We therefore used this reaction, with microwave irradiation as the heat source, to prepare new asymmetric tripodal ligands.
The desired product was isolated in high yield from the reaction using methanol (5 mL) as the solvent and NaBH3CN as a reductant, under microwave irradiation (60 °C, 300 W initial power) for 15 time (Table 1, entry 1). Further investigation showed that increasing the temperature to 70 or 80 °C (Table 1, entries 2 and 3) did not significantly changes the yield. When the reaction time and power were reduced (5 min and 100 W), lower yields were obtained (Table 1, entries 4 to 7). The optimum reaction time and power for synthesizing 5 were therefore 15 min and 300 W, respectively. When NaBH4 and NaBH(OAC)3 was used as reductant under the same conditions, the product was not obtained (Table 1, entries 8 and 9). Additionally, increasing the material to 10 mmol or 20 mmol led to minor decrease in the yield (Table 1, entries 10 and 11). However, the yields were still satisfactory.
| Entry | Time (min) | Temp (°C) | Power (W) | Yieldf (%) |
|---|---|---|---|---|
| a Yield of isolated yields.b NaBHCN3 as a reductant.c NaBH4 as a reductant.d NaBH(OAC)3 as a reductant.e 10 mmol of materials for each other.f 20 mmol of material for each other. | ||||
| 1b | 15 | 60 | 300 | 97 |
| 2b | 15 | 70 | 300 | 97 |
| 3b | 15 | 80 | 300 | 97 |
| 4b | 5 | 60 | 300 | NR |
| 5b | 10 | 60 | 300 | 78 |
| 6b | 15 | 60 | 100 | NR |
| 7b | 15 | 60 | 200 | 56 |
| 8c | 15 | 60 | 300 | NR |
| 9d | 15 | 60 | 300 | NR |
| 10b,e | 15 | 60 | 300 | 95 |
| 11b,f | 15 | 60 | 300 | 94 |
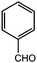
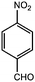
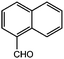
Based on the above results and our intention to us a green approach in this study, we performed the reaction using a shorter reaction time and less solvents, an almost quantitative yield was obtained (Table 1, entry 1). Having established the optimum conditions for our reaction, a series of aromatic aldehydes were tested in tripodal ligand synthesis of aromatic aldehydes and (2-hydro-xybenzyl-2-pyri-dylmethyl)amine (HBPA) at 300 W in methanol (Table 2). Polycyclic aromatic aldehydes gave the corresponding products in high yields (Table 2, entries 3 to 7).
The asymmetric tripodal ligands were analyzed by 1H and 13C NMR, FT-IR and HRM spectra (Fig. S4–S31 in the ESI†). Single crystals of the 3–5 suitable for X-ray diffraction, was obtained in 1 week by diethyl ether vapor diffusion into CHCl3 solution. The molecular structures of 3–5 are shown in Fig. 2. Crystal data, structure refinement details, and hydrogen bond interaction are listed in Tables S1–S3 and Fig. S1–S3 in ESI.†
 | ||
| Fig. 2 X-ray crystal structure and intramolecular hydrogen of 3–5; ellipsoids are draw at 30% probability level and H atoms with arbitrary size. | ||
The ligand structures belong to monoclinic space group, P21/c. All bond distances and angles are all within the normal ranges. There are two intramolecular hydrogen bonds, between the phenol and pyridine or the tertiary amine present in the crystal lattices: (a) phenol ring and corresponding tertiary amine (O1–H⋯N2 = 152.04°, 2.811 Å), and phenol ring and pyridyl ring (O1–H⋯N1 = 133.64°, 3.121 Å), in 3; (b) phenol ring and tertiary amine (O1–H⋯N2 = 152.04°, 2.811 Å and O1–H⋯N2 = 154.56°, 3.042 Å), and phenol ring and pyridyl ring (O1–H⋯N1 = 126.85°, 2.870 Å) in 4 and (c) phenol ring and tertiary amine (O1–H⋯N2 = 145.78°, 2.747 Å), and phenol ring and pyridyl (O1–H⋯N1 = 135.36°, 2.999 Å), in 5. These hydrogen bonding parameters are comparable to those reported for other tripodal ligands.11 The dihedral angles in 3 between the naphthyl group (C15–C24) and either the pyridyl ring (C1–C5–N1) or the phenol ring (C8–C13–O1) are 66.33° and 84.31°, respectively. The dihedral angles in 4 between the quinolyl group (C15–C24) and either the pyridyl ring (C1–C5–N1) or the phenol ring (C8–C13–O1) are 82.96° and 71.97°, respectively. The dihedral angles of 5 between the anthryl group (C15–C28) and either the pyridyl ring (C9–C13–N1) or the phenol ring (C1–C5–O1) are 69.79° and 46.69°, respectively. The phenol ring (C8–C13–O1) and the pyridyl rings (C9–C13–N1) have dihedral angles of 34.29°, 19.35° and 13.31° in 3–5.
In summary, a fast, atom-economic, high-yielding process for asymmetric tripodal ligand synthesis under microwave irradiation is described. Various of aldehydes can be used and the products are obtained in good to excellent yields. In future work, the title ligands will be used in the synthesis of metal complexes and research into their applications.
Acknowledgements
We thank the Natural Science Foundation of China (Grant no. 21172037), SRFDPHEC (Grant no. 201135141001), and the Natural Science Foundation of Fujian, China (Grant no. 2011J0ss1040) for financial support.Notes and references
- (a) S. Kisslinger, H. Kelm, A. Beitat, C. Wurtele, H. J. Kruger and S. Schindler, Inorg. Chim. Acta, 2011, 374, 540 CrossRef CAS PubMed; (b) M. Sarma, A. Kalita, P. Kumar, A. Singh and B. Mondal, J. Am. Chem. Soc., 2010, 132, 7846 CrossRef CAS PubMed; (c) M. Royzen and J. W. Canary, Polyhedron, 2013, 58, 85 CrossRef CAS PubMed; (d) B. K. Datta, C. Kar, A. Basu and G. Das, Tetrahedron Lett., 2013, 54, 771 CrossRef CAS PubMed; (e) J. S. Hart, G. S. Nichol and J. B. Love, Dalton Trans., 2012, 41, 5785 RSC; (f) P. C. Faggi, P. Bonaccorsi, L. M. Scolaro and M. C. Aversa, Eur. J. Inorg. Chem., 2013, 2013, 3412 CrossRef PubMed.
- (a) X. Chen, Q. Liu, H. B. Sun, X. Q. Yu and L. Pu, Tetrahedron Lett., 2010, 51, 2345 CrossRef CAS PubMed; (b) M. Irene, P. Maurizio, R. Luca, D. Mellmann, M. B. Henrik and G. Luca, Dalton Trans., 2013, 42, 2495 RSC.
- (a) U. Basu, I. Khan, A. Hussain, P. Kondaiah and A. R. Chakravary, Angew. Chem., Int. Ed., 2012, 51, 2658 CrossRef CAS PubMed; (b) B. Balaji, K. Somyajit, B. Banik, G. Nagaraju and A. R. Chakravarty, Inorg. Chim. Acta, 2013, 400, 142 CrossRef CAS PubMed; (c) T. K. Goswami, S. Gadadhar, B. Gole, A. A. Karande and A. R. Chakravarty, Eur. J. Med. Chem., 2013, 63, 800 CrossRef CAS PubMed.
- (a) S. S. Ashutosh and S. S. Sun, RSC Adv., 2012, 2, 9502 RSC; (b) L. Benhamou, H. Jaafar, A. Thibon, M. Lachkar and D. Mandon, Inorg. Chim. Acta, 2011, 373, 195 CrossRef CAS PubMed; (c) D. H. Gibson, J. Wu and M. S. Mashuta, Inorg. Chim. Acta, 2006, 359, 309 CrossRef CAS PubMed; (d) E. Palani, R. Palanisamy, H. Firasat and S. Malaichamy, RSC Adv., 2013, 3, 2171 RSC.
- (a) A. Mukherjee, F. Lloret and R. Mukherjee, Inorg. Chem., 2008, 47, 4471 CrossRef CAS PubMed; (b) M. S. Jesús, R. Bastida, A. Macías, H. Adams, D. E. Fenton, P. L. Paulo and L. Valencia, Polyhedron, 2010, 29, 2651 CrossRef PubMed; (c) A. Lucy, R. H. Mullice, L. P. Laye, N. J. B. Harding and J. A. Simon, New J. Chem., 2008, 32, 2140 RSC; (d) T. Cadenbach, E. Hevia, A. R. Kennedy, R. E. Mulvey, J. A. Pickrell and S. D. Robertson, Dalton Trans., 2012, 41, 10141 RSC; (e) T. Zhang, C. F. Chan, J. H. Hao, G. Law, W. K. Wong and K. L. Wong, RSC Adv., 2013, 3, 382 RSC.
- (a) Y. Hayashi, T. Kayatani, H. Sugimoto, M. Suzuki, J. K. Inomata, A. Uehara, J. Y. Mizutani, T. Kitagawa and Y. Maeda, J. Am. Chem. Soc., 1995, 117, 11220 CrossRef CAS; (b) J. W. Chen, X. Y. Wang, Y. G. Zhu, J. Lin, X. L. Yang, Y. Z. Li, Y. Lu and Z. J. Guo, Inorg. Chem., 2005, 44, 3422 CrossRef CAS PubMed; (c) F. Li, M. Wang, P. Li, T. T. Zhang and L. C. Sun, Inorg. Chem., 2007, 46, 9364 CrossRef CAS PubMed.
- (a) M. A. Surati, S. Jauhari and K. R. A. Desai, Appl. Sci. Res., 2012, 4, 645 Search PubMed; (b) V. R. Mudumala, C. S. R. Gangireddy and T. J. Yeon, RSC Adv., 2014, 4, 24089 RSC; (c) D. Vacchani and E. V. Eycken, Chem. Eur. J., 2013, 19, 1158 CrossRef PubMed; (d) M. Y. Liu, X. Wang, X. L. Sun and W. He, Tetrahedron Lett., 2014, 56, 2711 CrossRef PubMed; (e) W. Yin, Y. Ma, J. X. Xu and Y. F. Zhao, J. Org. Chem., 2006, 71, 4312 CrossRef CAS PubMed; (f) W. g. Duan, C. H. Chen, L. B. Jiang and G. H. Li, Carbohydr. Polym., 2008, 73, 582 CrossRef CAS PubMed; (g) M. Irene, P. Maurizio, R. Luca, D. J. Mellmann, M. B. Henrik and G. Luca, Dalton Trans., 2013, 42, 2495 RSC.
- (a) L. Dimitris and G. K. Christoforos, RSC Adv., 2013, 3, 4496 RSC; (b) A. M. Sarotti, M. M. Joullié, R. A. Spanevello and A. G. Suárez, Org. Lett., 2006, 8, 5561 CrossRef CAS PubMed; (c) D. D. Young, J. Nichols, R. M. Kelly and A. Deiters, J. Am. Chem. Soc., 2008, 130, 10048 CrossRef CAS PubMed; (d) A. K. Greene and L. T. Scott, J. Org. Chem., 2013, 78, 2139 CrossRef CAS PubMed; (e) S. Horikoshi and N. Serpone, Microwaves Org. Synth., 2013, 1, 377 Search PubMed; (f) D. N. Pansare and D. B. Shinde, Tetrahedron Lett., 2014, 55, 1107 CrossRef CAS PubMed.
- (a) S. A. De-Silva, M. A. Whitener, D. E. Baron, A. Zavaleta, E. V. Isidor and O. Allam, Acta Crystallogr., 1998, C54, 1117 CAS; (b) H. Lee, H. S. Lee, J. H. Reibenspies and R. D. Hancock, Inorg. Chem., 2012, 51, 10904 CrossRef CAS PubMed; (c) H. Woo, S. Cho, Y. Han, W. S. Chae, D. R. Ahn, Y. M. You and W. Nam, J. Am. Chem. Soc., 2013, 135, 4771 CrossRef CAS PubMed; (d) M. Ganeshpandian, R. Loganathan, E. Suresh, A. Riyasdeen, M. A. Akbarsha and M. Palaniandavar, Dalton Trans., 2014, 43, 1203 RSC.
- (a) B. M. Trost, Angew. Chem., Int. Ed., 1995, 34, 259 CrossRef CAS PubMed; (b) J. Fan, Q. Y. Yang, G. J. He, X. G. Xie, H. Y. Zhu, Y. Jin and J. Lin, RSC Adv., 2014, 4, 28852 RSC.
- (a) A. Neves, C. N. Verani, M. A. Brito, I. Vencato, A. Mangrich, G. Oliva, D. D. H. F. Souza and A. A. Batista, Inorg. Chim. Acta, 1999, 290, 207 CrossRef CAS; (b) Y. C. Shimazaki, S. Huth, A. Odani and O. Yamauchi, Angew. Chem., Int. Ed., 2000, 39, 1666 CrossRef CAS; (c) E. J. Song, J. Kang, G. R. You, G. J. Park, Y. Kim, S. J. Kim, C. Kim and R. G. Harrison, Dalton Trans., 2013, 42, 15514 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Full experimental procedures and data. CCDC 966462, 966463 and 966464. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra07346k |
| This journal is © The Royal Society of Chemistry 2014 |