Theoretical study of the thermoelectric properties of SiGe nanotubes
Abstract
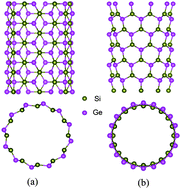
The thermoelectric properties of two typical SiGe nanotubes are investigated using a combination of density functional theory, Boltzmann transport theory, and molecular dynamics simulations. Unlike carbon nanotubes, these SiGe nanotubes tend to have gear-like geometry, and both the (6, 6) and (10, 0) tubes are semiconducting with direct band gaps. The calculated Seebeck coefficients as well as the relaxation time of these SiGe nanotubes are significantly larger than those of bulk thermoelectric materials. Together with smaller lattice thermal conductivity caused by phonon boundary and alloy scattering, these SiGe nanotubes can exhibit very good thermoelectric performance. Moreover, there are strong chirality, temperature and diameter dependences of the ZT values, which can be optimized to 4.9 at room temperature and further enhanced to 5.4 at 400 K for the armchair (6, 6) tube.

 Please wait while we load your content...
Please wait while we load your content...