Lanthano-phosphotungstate: A water soluble and reusable catalyst for oxidation of alcohols using H2O2 as an oxidant†
Mukesh Kumar Saini,
Rakesh Gupta,
Swati Parbhakar,
Surendra Singh* and
Firasat Hussain*
Department of Chemistry, University of Delhi, Delhi, India. E-mail: fhussain@chemistry.du.ac.in; Fax: +91 11 2766 6605; Tel: +91 11 27666930
First published on 12th August 2014
Abstract
A series of water soluble lanthano-phosphotungstate K11[Ln(PW11O39)2]·xH2O [Ln = Pr(III), Nd(III), Eu(III), Gd(III), Tb(III), Dy(III), Ho(III), Er(III), Tm(III) and Yb(III)] catalysts were synthesized. These catalysts (0.24 mol%) were screened for oxidation of primary and secondary alcohols using H2O2 as an oxidant in water. The Pr-POM catalyst retained its activity after being recycled and reused upto five cycles.
Polyoxometalates (POMs), inorganic metal-oxo nanoclusters, have gained much importance in research due to their versatile properties. These are basically formed by transition metal oxides in their high oxidation states (mainly Mo, W, V, Nb and Ta). The growing interest in POMs undoubtedly reflects their discrete, molecular nature isolated from a solution-based approach, either from aqueous or non-aqueous media combined with highly flexible and tunable molecular composition, size, shape, charge density, redox potentials, acidity, solubility characteristics and high thermal stability. As a result, these nanoclusters can be used over a wide range of applications including homogeneous and heterogeneous oxidation catalysis, photocatalysis, biomedicine, materials science, data storage, sensors, and nano-biotechnology.1
Alcohol oxidation to carbonyl compounds is one of the most important transformations in organic chemistry. Hydrogen peroxide and molecular oxygen as most preferable eco-friendly oxidants used in these oxidation processes. For the last three decades, varieties of POMs were used as effective catalysts for the oxidation of various alcohols. Most of the reports till date on alcohol oxidation with POM catalysts have used non-ecofriendly organic solvents2 while some have utilized under solvent free conditions.3 To overcome the use of organic solvents and catalyst recovery problems, many researchers explored the use of green synthetic oxidation processes using water as a solvent. In addition water soluble POM catalysts were also explored by many researchers for the effective oxidation of various alcohol compounds e.g. monolacunary K8[β-SiW11O39]·14H2O, K8[BW11O39H]·13H2O dilacunary K8[γ-SiW10O36]·13H2O and trilacunary [SiW9O34]10− in conjugation with dioctadecyl dimethyl ammonium anion, zinc and vanadium substituted POMs.4
Lanthanoid containing POM catalysts have been less explored for the oxidation processes. Kera and co-workers studied the oxidation of cyclohexanol in homogeneous medium using [CeIVW10O36]8−, [NdIIIW10O36]9− and [SmIIIW10O36]9− heteropolytungsto-lanthanate5 anions as a catalyst, in the presence of H2O2 oxidant. Later, they also reported the catalytic behaviour of a series of lanthanide decatungstates (LnIIIW10O36)9− (Ln = La–Yb)6 and {C5H5N(CH2)15CH3}7H2HoIIIW10O36 POM catalyst for the oxidation of alcohols and olefins, using a biphasic (CHCl3–aqueous H2O2) solvent system.7 Griffith et al. also studied the use of lanthano-polyoxotungstates [Ln{PW11O39}2]11− (Ln = Y, La, Ce, Pr, Sm, Tb, Yb)8 and [MIV{PW11O39}2]10− POM catalysts for oxidation of benzyl alcohol and 2-octanol using biphasic systems (t-butanol/aqueous and benzene/aqueous) with [N(C6H13)4]Cl as a phase transfer agent.9 They concluded that lanthano-polyoxotungstates did not retain their identity after the catalysis, and transformed to the [(PO4){WO(O2)2}4]3−, [(PO4){WO(O2)2{WO(O2)2(H2O)}]3− and [(PO3(OH)){WO(O2)2}2]2− type active species.10 These active species were previously reported as catalyst for the alcohol oxidation processes.11 Recently, Zhao et al. studied the selective oxidation of various substrates (alkenes, alkenols, sulphides, silane and alcohols) using DA11[La(PW11O39)2] (DA = decyltrimethylammonium cation) POM catalyst, and H2O2 oxidant in acetonitrile.12
Herein, we report the synthesis of a series of water-soluble lanthano-phosphotungstates (POMs) as an oxidation catalyst for various alcohols in the presence of H2O2 oxidant. All the POMs K11[Ln(PW11O39)2]·xH2O [Ln = Pr(III), Nd(III), Eu(III), Gd(III), Tb(III), Dy(III), Ho(III), Er(III), Tm(III) and Yb(III)] were synthesized by a single step reaction of lanthanoid cations with [PW11O39]11− anion in KOAc buffer at pH 4.8 (see ESI† for detailed experimental section). All isolated compounds were structurally characterized by 31P-NMR, TGA analysis and FT-IR spectroscopy.13
The oxidation of 1-phenylethanol (2 mmol) was carried out using Pr-POM catalysts (0.24 mol%), in the presence of water (0.4 mL) and 30% aq. H2O2 (4.0 mmol) at different temperature 60, 70, 80 and 90 °C. We observed that increase in temperature from 60–90 °C, the conversion of 1-phenylethanol to acetophenone was increased from 40 to >99% (see Table 1). In addition we have also increased the amount of H2O2 (5.0 eq.) which resulted in excellent conversion >99% of the reactant to the product after 2 h. We have also decreased the loading of the catalysts upto 0.12 mol%, which was sufficient to convert >99% of 1-phenylethanol to acetophenone after 6 h. We also envisaged the praseodymium salt and monolacunary ligand (PW11O39) independently as a catalyst for the oxidation of 1-phenyl ethanol and found to be less active then their complex (Pr-POM) (see Table 1 entries 7 and 8).
| Entry | Reaction temperature (°C) | Catalysts loading (mol%) | Conversionb (%) | Reaction time (h) |
|---|---|---|---|---|
| a Reaction condition: catalyst (0.12–0.24 mol%), 1-phenylethanol (2 mmol), H2O2 (4 mmol) were taken in water (0.4 mL) and heated at specified temperature and time.b Conversion was determined by gas chromatography.c H2O2 was used as 5 equivalent.d Pr(NO3)3·6H2O used as a catalyst.e K7[PW11O39]·14H2O used as a catalyst.f Reaction carried out for 3 h. | ||||
| 1 | 60 | 0.24 | 42 | 3 |
| 2 | 70 | 0.24 | 68 | 3 |
| 3 | 80 | 0.24 | 80 | 3 |
| 4 | 90 | 0.24 | >99 | 3 |
| 5 | 90 | 0.12 | >99 | 6 |
| 6 | 90 | 0.24 | 100c | 2 |
| 7 | 90 | 5.0 | 38d | 6 |
| 8 | 90 | 0.24 | 78e | 3 |
| 9 | 90 | 0.12 | 90f | 3 |
We have screened different catalysts Pr–Yb POMs (0.24 mol%) for the oxidation of 1-phenylethanol to acetophenone (see Table S1†). The conversions of product vs. different catalysts are shown in Fig. 1.
The conversion of 1-phenylethanol to acetophenone was observed for all the catalysts from 76 to >99% in 3 h (see ESI – Table 1†). The Pr-POM catalyst was more active as compared to the other lanthanoid containing POM catalysts.
The turn over frequency (TOF) of the catalyst K11[Pr(PW11O39)2]·22H2O (0.24 mol%) for the oxidation of 1-phenyl ethanol, was found to be 137.5 h−1. We have also compared the TOF of the reported catalysts in the literature and the catalysts found to be more active than other catalysts and comparable to the zinc substituted POMs (TOF 138.3–156.7 h−1) (see ESI – Table 2†).
The chemical kinetics for the 1-phenylethanol oxidation reaction has been demonstrated as a function of time for the Pr-POM. The data plotted showed that the oxidation reaction is of first order (R2 = 0.99574) with respect to 1-phenylethanol (see ESI – Fig. 1†).
To establish the versatility of our POMs catalyst, we also studied oxidation of various alcohols namely 1-(4-bromophenyl)ethanol, 1-(3-bromophenyl)ethanol, 1-(3-nitrophenyl)ethanol, benzyl alcohol, benzhydrol, cyclopentanol, cyclohexanol and n-butanol at 90 °C using 0.24–0.6 mol% catalysts, and 2–5 equivalent of H2O2 (Table 2).
| Entry | Substrate | Product | Conversion (%) | Time (h) |
|---|---|---|---|---|
| a Reaction condition: Catalyst (0.24 mol%), alcohols (2 mmol), H2O2 (4 mmol) were taken in water (0.4 mL) and heated at 90 °C.b H2O2 was used as (10 mmol).c Catalyst (0.6 mol%), and H2O2 (10 mmol) was used.d Reaction was carried out under inert (N2) atmosphere. | ||||
| 1 |  |
 |
38 | 14 |
| 2 | 66b | 14 | ||
| 3 | 95c | 24 | ||
| 4 |  |
 |
89c | 24 |
| 5 |  |
 |
51 | 14 |
| 6 | 93c | 8 | ||
| 7 |  |
 |
70 | 3 |
 |
12 | |||
| 8 |  |
 |
42 | 8 |
| 9 | 90c | 14 | ||
| 10 |  |
 |
>99 | 3 |
| 11 | 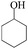 |
 |
>99 | 3 |
| 12 | n-Butanol | Butyric acid | >99 | 8 |
| 13 | n-Butanol | Butyraldehyde | 83d | 3 |
| 14 | n-Butanol | Butyraldehyde | 37 | 3 |
| Butyric acid | 48 | |||
| 15 | 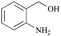 |
 |
21b | 6 |
 |
23b | |||
| Impurity | 45b | |||
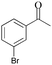
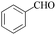
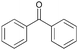
The oxidation of 1-(4-bromophenyl) ethanol gave 38% conversion of 1-(4-bromophenyl) ethanone (Table 2, entry 1). By increasing the amount of oxidant to 5 equiv. the conversion was improved (Table 2, entry 2). Furthermore, on increasing the catalysts loading from 0.24 to 0.6 mol% the conversion increased to 95% after 24 h, whereas 1-(3-bromophenyl) ethanol shows 89% conversion (Table 2, entries 3 and 4). On oxidation of 1-(3-nitrophenyl) ethanol, 51% conversion was observed after 14 h, which was improved upto 93% by increasing the catalysts loading (0.6 mol%) and H2O2 (5 equiv.) (Table 2, entries 5 and 6). The oxidation of benzyl alcohol gave benzaldehyde (70%) and benzoic acid (12%) after 3 h (Table 2, entry 7). The oxidation of benzhydrol converted to benzophenone 42% after 8 h and 90% after 14 h by increasing catalyst loading from 0.24 to 0.6 mol% (Table 2, entry 8 and 9). The cyclic alcohols cyclohexanol and cyclopentanol show excellent conversion (>99%) to the corresponding ketones after 3 h (Table 2, entries 10 and 11). We have also conducted the oxidation of n-butanol which gave butyric acid >99% after 8 h (Table 2, entry 12). As butyraldehyde is easily oxidized in air, therefore we performed the oxidation of n-butanol under N2-atmosphere, gave exclusively butyraldehyde as a product in 83% conversion after 3 h (Table 2, entries 13). The identical reaction in absence of N2-atmosphere gave butyraldehyde (48%) and butyric acid (37%) (Table 2, entry 14). The oxidation of 2-amino-benzylalcohol was carried out to know the chemo-selectivity of catalytic process. We found the process not to be chemo-selective, and gave 2-nitro-benzylalcohol, 2-nitrobenzoic acid, and other impurities as products, which indicates that the amino group is oxidized quickly, and then to the corresponding primary alcohol (Table 2, entry 15).
The product was extracted with diethyl ether and the catalysts present in the aqueous medium were used for next catalytic cycle.4j The Pr-POM shows consistent activity for oxidation of 1-phenylethanol to acetophenone (see Table 3). After five catalytic cycles, Pr-POM was recovered on addition of ethanol to the aqueous medium and FTIR spectra was recorded on KBr pellets, which shows no change in the POM structure (see ESI for detailed procedure of catalyst recovery, Fig. S3†).
| Entry | Catalyst cycle | Conversion (%) |
|---|---|---|
| a Reaction conditions: Pr-POM (0.24 mol%), 1-phenylethanol (2 mmol), H2O2 (4 mmol) were taken in water (0.4 mL) and heated at 90 °C for 3 h. | ||
| 1 | 1 | >99 |
| 2 | 2 | >99 |
| 3 | 3 | 95 |
| 4 | 4 | 98 |
| 5 | 5 | 90 |
In conclusion, we have reported the synthesis of a series of water-soluble lanthano-phosphotungstates in a single step reaction and also investigated the catalysis for the oxidation of alcohols using environmental friendly oxidant (H2O2). 1-Phenylethanol was oxidized to acetophenone with >99% conversion and the non-aromatic cyclic alcohols shows excellent conversion to corresponding ketones for Pr(III)-POM. The Pr(III)-POM catalyst was also envisaged for the oxidation of substituted 1-phenyl ethanol. The primary alcohols were also oxidized to corresponding aldehydes in good yields in presence of inert atmosphere. The catalysts could be recovered and reused up to five catalytic cycles without the loss of its activity.
Acknowledgements
F. H. thanks University of Delhi and CSIR-New Delhi (Project no. 01(2712)/13/EMR-II) for research support. We are grateful to Department of Chemistry, USIC, M. Tech. NSNT, University of Delhi for providing instrument facility. F. H. thanks Dr E. Dapurkar, Tata Chemicals Ltd. Pune, India for helpful discussion. This work is dedicated to Prof. Ulrich Kortz.Notes and references
- (a) M. T. Pope, Heteropoly and Isopoly Oxometalates, Springer-Verlag, Berlin, 1983 Search PubMed; (b) C. L. Hill and C. M. Prosser-McCartha, Coord. Chem. Rev., 1995, 143, 407 CrossRef CAS; (c) M. T. Pope and A. Müller, Angew. Chem., Int. Ed. Engl., 1991, 30, 34 CrossRef PubMed; (d) Chem. Rev., 1998, 98, Special Issue on Polyoxometalates, ed. C. L. Hill Search PubMed; (e) A. Müller and S. Roy, Coord. Chem. Rev., 2003, 245, 153 CrossRef; (f) E. Coronado and P. Day, Chem. Rev., 2004, 104, 5419 CrossRef CAS PubMed; (g) L. Cronin, in Compr. Coord. Chem. II, ed. J. A McCleverty and T. J. Meyer, Elsevier, Amsterdam, 2004, vol. 7, pp. 1–57 Search PubMed; (h) C. L. Hill, J. Mol. Catal. A: Chem., 2007, 262, 2–6 CrossRef CAS PubMed; (i) U. Kortz, A. Müller, J. van Slageren, J. Schnack, N. S. Dalal and M. Dressel, Coord. Chem. Rev., 2009, 253, 2315 CrossRef CAS PubMed; (j) B. Hasenknopf, Front. Biosci., 2005, 10, 275 CrossRef CAS PubMed; (k) L. Zhongfeng, L. Weisheng, L. Xiaojing, P. Fengkui, L. Yingxia and L. Hao, Magn. Reson. Imaging, 2007, 25, 412 CrossRef PubMed; (l) Eur. J. Inorg. Chem., 2009, 34, Issue dedicated to Polyoxometalates; Guest ed. U. Kortz Search PubMed.
- (a) M. V. Vasylyev and R. Neumann, J. Am. Chem. Soc., 2004, 126, 884 CrossRef CAS PubMed; (b) M. Carraro, L. Sandei, A. Sartorel, G. Scorrano and M. Bonchio, Org. Lett., 2006, 8, 3671 CrossRef CAS PubMed; (c) F. F. Bamoharram, M. Roshani, M. M. Heravi and S. Safaie, Phosphorus, Sulfur Silicon Relat. Elem., 2006, 181, 2833 CrossRef CAS; (d) Z. H. Weng, J. Y. Wang and X. G. Jian, Catal. Commun., 2008, 9, 1688 CrossRef CAS PubMed; (e) X. Lang, Z. Li and C. Xia, Synth. Commun., 2008, 38, 1610 CrossRef CAS; (f) B. G. Donoeva, T. A. Trubitsina, G. M. Maksimov, R. I. Maksimovskaya and O. A. Kholdeeva, Eur. J. Inorg. Chem., 2009, 5142 CrossRef CAS PubMed; (g) O. A. Kholdeeva, B. G. Donoeva, T. A. Trubitsina, G. Al-Kadamany and U. Kortz, Eur. J. Inorg. Chem., 2009, 5134 CrossRef CAS PubMed; (h) B. G. Donoeva, T. A. Trubitsyna, G. Al-Kadamany, U. Kortz and O. A. Kholdeeva, Kinet. Catal., 2010, 51, 816 CrossRef CAS; (i) Y. Kikukawa, K. Yamaguchi and N. Mizuno, Inorg. Chem., 2010, 49, 8194 (Angew. Chem., Int. Ed., 2010, 49, 6096) CrossRef CAS PubMed; (j) P. Tundo, G. P. Romanelli, P. G. Vazquez and F. Arico, Catal. Commun., 2010, 11, 1181 CrossRef CAS PubMed; (k) Y. Ding and W. Zhao, J. Mol. Catal. A: Chem., 2011, 337, 45 CrossRef CAS PubMed; (l) Z. Nadealian, V. Mirkhani, B. Yadollahi, M. Moghadam, S. Tangestaninejad and I. Mohammadpoor-Baltork, J. Coord. Chem., 2012, 65, 1071 CrossRef CAS.
- (a) R. H. Ingle, N. K. K. Raj and P. Manikandan, J. Mol. Catal. A: Chem., 2007, 262, 52 CrossRef CAS PubMed; (b) S. J. Zhang, G. D. Zhao, S. Gao, Z. W. Xi and J. Xu, J. Mol. Catal. A: Chem., 2008, 289, 22 CrossRef CAS PubMed; (c) W. Tian, Y. Hou, X. G. Wang, B. Lu, J. X. Zhao and Q. H. Cai, Chin. J. Chem., 2012, 30, 433 CrossRef CAS PubMed.
- (a) D. Sloboda-Rozner, P. L. Alsters and R. Neumann, J. Am. Chem. Soc., 2003, 125, 5280 CrossRef PubMed; (b) D. Sloboda-Rozner, P. Witte, P. L. Alsters and R. Neumann, Adv. Synth. Catal., 2004, 346, 339 CrossRef CAS PubMed; (c) J. Wang, L. Yan, G. Li, X. Wang, Y. Ding and J. Suo, Tetrahedron Lett., 2005, 46, 7023 CrossRef CAS PubMed; (d) P. Maity, D. Mukesh, S. Bhaduri and G. K. Lahiri, J. Chem. Sci., 2009, 121, 377 CrossRef CAS PubMed; (e) B. Ma, Y. Zhang, Y. Ding and W. Zhao, Catal. Commun., 2010, 11, 853 CrossRef CAS PubMed; (f) W. Zhao, Y. Zhang, B. Ma, Y. Ding and W. Y. Qiu, Catal. Commun., 2010, 11, 527 CrossRef CAS PubMed; (g) Y. Ding, W. Zhao, B. Ma and W. Y. Qiu, Can. J. Chem., 2011, 89, 13 CrossRef CAS PubMed; (h) Y. Ding, W. Zhao, Y. S. A. Zhang, B. Ma and W. Y. Qiu, React. Kinet., Mech. Catal., 2011, 102, 85 CrossRef CAS; (i) W. Zhao, Y. Ding, B. Ma and W. Y. Qiu, Synth. Commun., 2012, 42, 554 CrossRef CAS; (j) Z. Zhang, Q. Zhu and Y. Ding, Synth. Commun., 2013, 43, 1211 CrossRef CAS.
- R. Shiozaki, H. Goto and Y. Kera, Bull. Chem. Soc. Jpn., 1993, 66, 2790 CrossRef CAS.
- R. Shiozaki, A. Inagaki, A. Ozaki, H. Kominami, S. Yamaguchi, J. Ichihara and Y. Kera, J. Alloys Compd., 1997, 261, 132 CrossRef CAS.
- R. Siozaki, A. Inagaki, H. Kominami, S. Yamaguchi, J. Ichihara and Y. Kera, J. Mol. Catal. A: Chem., 1997, 124, 29 CrossRef CAS.
- W. P. Griffith, R. G. H. Moreea and H. I. S. Nogueira, Polyhedron, 1996, 15, 3493 CrossRef CAS.
- N. M. Gresley, W. P. Griffith, A. C. Laemmel, H. I. S. Nogueira and B. C. Parkin, J. Mol. Catal. A: Chem., 1997, 117, 185 CrossRef CAS.
- W. P. Griffith, N. Morley-Smith, H. I. S. Nogueira, A. G. F. Shoair, M. Suriaatmaja, A. J. P. White and D. J. Williams, J. Organomet. Chem., 2000, 607, 146 CrossRef CAS.
- C. Venturello and M. Gambaro, J. Org. Chem., 1991, 56, 5924 CrossRef CAS.
- S. Zhao, Y. Q. Jia and Y. F. Song, Appl. Catal., A, 2013, 453, 188 CrossRef CAS PubMed.
- R. Gupta, M. K. Saini, F. Doungmene, P. de Oliviera and F. Hussain, Dalton Trans., 2014, 43, 8290 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra07302a |
| This journal is © The Royal Society of Chemistry 2014 |


