From micron to nano-curcumin by sophorolipid co-processing: highly enhanced bioavailability, fluorescence, and anti-cancer efficacy†
Abstract
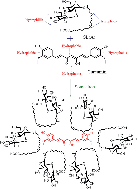
Co-sonication of curcumin and acidic sophorolipid in aqueous solution is shown to lead to a dramatic enhancement of curcumin bioavailability through size reduction and encapsulation. The interaction between the two is studied and discussed based on optical absorption, photoluminescence, dynamic light scattering (DLS), zeta potential, FE-SEM, TEM, Infrared spectroscopy and X-ray diffraction measurements. The cytotoxicity effects of curcumin on breast cancer cell lines, MCF-7 and MDA-MB-231, are shown to be significantly enhanced by the formation of its complex with sophorolipid. The relative cytotoxicity of curcumin with its SL(A) complex is more due to the presence of the glucose moiety. The results further suggest that sophorolipid based formulations, which solubilize and nano-encapsulate curcumin after lipid digestion, show great potential for curcumin cell entry.

 Please wait while we load your content...
Please wait while we load your content...