Optimization of ultrasound-assisted extraction of protein from egg white using response surface methodology (RSM) and its proteomic study by MALDI-TOF-MS
Juanni He,
Hongtao Yan* and
Chunlei Fan
Key Laboratory of Synthetic and Natural Functional Molecule Chemistry of Ministry of Education, College of Chemistry and Materials Science, Northwest University, Xi'an 710069, China. E-mail: htyan@nwu.edu.cn; Fax: +86-29-88302604; Tel: +86-29-88302604
First published on 28th August 2014
Abstract
The presented work proposes an approach based on proteomic techniques to identify proteins in egg white. Response surface methodology (RSM) with Box–Behnken design (BBD) was used to optimize the ultrasound-assisted extraction process of proteins from the egg white. The effects of extraction parameters, including trifluoroacetic acid (TFA) concentration, ultrasonic power and ultrasonic time on the extraction process of proteins were evaluated. The optimized extraction parameters were trifluoroacetic acid (TFA) concentration, 0.53% (v/v); ultrasonic power, 50% and ultrasonic time, 40 min. The effects of three factors (trypsin volume, pH of tryptic digestion and incubation time) on the tryptic digestion process of extracted proteins were investigated. The results showed that the optimized tryptic digestion conditions were as follows: volume of 10 μg mL−1 trypsin, 8 μL; pH, 7.0 and incubation time, 16 h. Under the optimized conditions, the peptide mixture was analysed using matrix-assisted laser desorption/ionization-time of flight-mass spectrometry (MALDI-TOF-MS). The methodology was successfully applied to free-range and barn-raised chicken egg white for protein identification. The results showed that 23 proteins and 29 proteins were identified in free-range chicken and barn-raised chicken egg white, respectively. Among them, the collagen peptides were found in egg white for the first time.
1. Introduction
Chicken egg is an inexpensive source of high quality protein in the human diet, which is composed of water (75%), proteins (12%), and lipids (12%), as well as carbohydrates etc. egg white, a high-viscosity solution, in particular, has been used as a raw ingredient for decades in the food industry.1,2 Egg polyfunctionality in food systems is correlated, to a high extent, with its chemical composition and more specifically with its protein content. Besides that, some of the minor components could be interesting for nonfood applications, such as health uses.3Many researches have been conducted to identify the novel proteins and their isoforms in egg white. Egg proteins were previously studied using classical biochemical techniques such as chromatographic and electrophoretic separations, together with Edman sequence analysis. Up to 1989, only 13 proteins were usually referenced in egg white, some of which were not even fully characterized. It is difficult to identify the proteins of egg white because of their different molecular weights and pI values, different concentration levels, diversified polymorphic forms as well as their complex matrices. With the advent of new ionization techniques of matrix-assisted laser desorption/ionization (MALDI), MS has become a powerful tool in structural characterization of large biomolecules,4–6 and it has high selectivity and sensitivity, tolerance toward contaminants and provides fast analysis. Proteomic methodologies for identification of proteins by MS can provide reliable identification of the different proteins in comparison with other methods and have gained more attention during recent years.7 Guérin-Dubiard et al.,8 via 2-D electrophoresis and ESI LC-MS/MS, identified 16 proteins in egg white in which Tenp and VMO-1 were detected for the first time. Raikos et al.,9 via SDS-PAGE and 2-D PAGE, combined with matrix-assisted laser desorption/ionization-time of flight-mass spectrometry (MALDI-TOF-MS), identified five proteins, namely ovalbumin, ovotransferrin, clusterin, activin receptor IIA and the hypothetical protein FLJ10305. A major breakthrough came with the work of Mann10 who Using 1-D PAGE and LC-MS/MS and MS3 identified 78 proteins in chicken egg white, 54 of which were identified in egg white for the first time. D'Ambrosio et al.11 have doubled the number of proteins (to 148) found in egg white by exploiting a peptide ligand libraries technology. Omana et al.12 determined the changes of proteins in egg white from white-shell eggs during storage to further understand the biochemical basis of egg white thinning. Egg white thinning is regarded as a sign of loss of quality that leads to staleness.13 Some researches compared free-range chicken egg varieties and barn-raised chicken egg varieties in xanthophyll composition,14 whipping capacity and foam consistency,15 levels of PCDD/Fs, PCBs and PBDEs,16 and so on. Proteins are the main components in egg white except water, however, to date, many of the proteins located in eggs from different housing systems remain uncharacterised, if not unknown. The main objective of the present study was to evaluate the proteins in egg white from free-range and barn-raised chicken.
The key steps in proteomic studies by MS methods usually involve extraction of proteins in complex mixtures. H. C. Lee et al.17 optimized extraction conditions of protein and starch from lentils via single-factor experiment which showed no significant difference was observed for extracted protein at the various extraction pH and temperature. Response surface methodology (RSM), an effective statistical technique for optimizing complex extraction processes, can reduce the number of experimental trials and evaluate the mutual interactions between multiple parameters. Box–Behnken design (BBD) is an RSM model that examines the relationships between several explanatory variables and one or more response variables.18–20 In this current study, we have used MALDI-TOF-MS in an attempt to identify the proteins in the free-range chicken and barn-raised chicken egg white. Methods for response surface methodology (RSM) with Box–Behnken design (BBD) have been used to optimize the conditions of ultrasound-assisted extraction of protein from egg white. Under the optimized conditions, the peptide mass fingerprints (PMF) of the proteins of free-range chicken and barn-raised chicken egg white were determined by MALDI-TOF-MS.
2. Experimental section
2.1. Reagents and materials
All of chemicals used in the current study were purchased as the analytical reagent grade. Deionized water was used throughout whole process.Sequencing grade trypsin was purchased from Promega (Madison, WI, USA). α-Cyano-4-hydroxycinnamic acid (CHCA), dithiothreitol (DTT) was purchased from Merck (Darmstadt, Germany). Iodoacetamide (IAA) was purchased from Amresco (Ohio, USA). Trifluoroacetic acid (TFA) was purchased from Aladin (Shanghai, China). Urea and ammonium hydrogen carbonate were purchased from Xi'an Chemical Reagent Plant (Xi'an, China).
Free range eggs were obtained from the countryside of Weinan city, Shaanxi province, China. Barn-raised eggs were obtained from the local supermarket of Xi'an.
2.2. Sample preparation
Free range eggs and barn-raised eggs, both less than 5 days after being laid, were used. They were both stored at 4 °C. The egg white was drawn from eggs with small glass sucker (0.4 mm diameter) and collected. The egg white was spread-out as thin film across several microscope glass slides and evaporated at ambient temperature (25 °C). Every effort was made to prevent contamination of samples during the sampling process.112.3. Protein extraction
The proteins were extracted from the samples (40 mg) using 0.6 mL of 0.6% (v/v) TFA. The resulting extracts were subjected to an ultrasonic bath treatment for 45 min. The mixtures were then centrifuged 15 min at 4000×g and the supernatant was collected.The protein concentration of the supernatant (soluble protein extracts) was determined using Bradford's method.21,22
2.4. Experimental design for protein extraction
To further optimize the extraction conditions, a three-variable-three-level BBD was employed in this experiment.23–25 The three independent variables were TFA concentration (v/v), ultrasonic power (%) and ultrasonic time (min), respectively. Each variable was set at three levels. A total of 15 experiments were designed according to BBD. Each experiment was performed in triplicate and the average protein concentration (mg mL−1) was taken as the response.2.5. Enzymatic digestion
The procedure used for the hydrolysis of the extracted proteins in the current study has been described in detail elsewhere in the literature26,27 with some improvement here. In brief, the 150 μL of protein solution was denatured in 0.5 mL of 40 mM NH4HCO3 (pH 7.8) by urea at a final concentration of 8 mol L−1. Then, it was reduced with 12 mM dithiothreitol (final concentration) and incubated at 50 °C for 3 h. The alkylation was achieved with iodoacetamide at 300 mM final concentration, and the reaction was carried out in the dark at room temperature for 30 min. This sample was dialyzed against deionized water and phosphate buffer solution (PBS, pH 7.0) overnight. Finally, 8 μL of 10 μg mL−1 trypsin was added to the prepared 50 μL of protein solution and the mixture was incubated at 37 °C for 16 h. The solution was then stored at −20 °C until further analysis.2.6. MALDI-TOF-MS and database search
The MW (molecular weights) of peptides was measured using an AutoflexIII Smartbean MALDI-TOF mass spectrometer (Bruker, Germany) equipped with a standard nitrogen laser (λ 337 nm) in linear mode. Each experiment started with recalibration of the mass spectrometer with a commercial standard of peptide mixture (Pepmix, Bruker). A 1 μL aliquot of the peptide mixture was mixed in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (v/v) with the matrix (CHCA) solution (10 mg mL−1 in ACN
1 ratio (v/v) with the matrix (CHCA) solution (10 mg mL−1 in ACN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TFA 0.1%, 50
TFA 0.1%, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50). The resulting mixture was spotted on the stainless steel MALDI target and was leaved for air drying at room temperature before MALDI-TOF-MS analysis. At least 300 laser shots were collected for each spectrum.
50). The resulting mixture was spotted on the stainless steel MALDI target and was leaved for air drying at room temperature before MALDI-TOF-MS analysis. At least 300 laser shots were collected for each spectrum.
All of the searches of the PMF spectra were obtained against SwissProt non-redundant database (SwissProt_2013.6.27) and carried out using MS-Fit search engine, accessible at http://prospector.ucsf.edu/.27
3. Results and discussion
3.1. Single-factor optimization of protein extraction condition
It is important for identification of proteins to select the appropriate methods and conditions of protein extraction in the experiment. The extraction of protein using concentrated HCl, NaOH, or NH3 has been proposed,28–30 but these reagents often lead to selective degradation of amino acids, which affects the identification of protein. TFA is shown to be a promising extraction agent in the identification of protein.26,31 Compared to other methods,32,33 TFA-ultrasound-assisted extraction can provide the total proteins, it has such advantages as simple and rapid operation, good repeatability, low consumption chemical reagents. It can minimize the sample loss and avoid the risk of sample contaminant result from in the digestion or derivatisation. In this current work, TFA-ultrasound-assisted extraction was selected to extract protein from chicken egg white. The effects of TFA concentration, ultrasonic power and ultrasonic time on ultrasound-assisted extraction of protein from egg white were investigated (Fig. 1). As shown in Fig. 1A, protein concentration increased with TFA concentration increased until 0.6% (v/v), and began to decrease. A certain concentration of TFA can dissolve protein well but high concentration of TFA maybe lead to protein denaturation. The effects of ultrasonic power (Fig. 1B) and ultrasonic time (Fig. 1C) showed the similar fluctuation trend to TFA concentration. Protein concentration increased accompanying increases of the two variables. When ultrasonic power and ultrasonic time increased respectively over 70% and 45 min, protein concentration reduced as a result of hydrophilic interactions of some globulin proteins and protein denaturation or hydrolysate.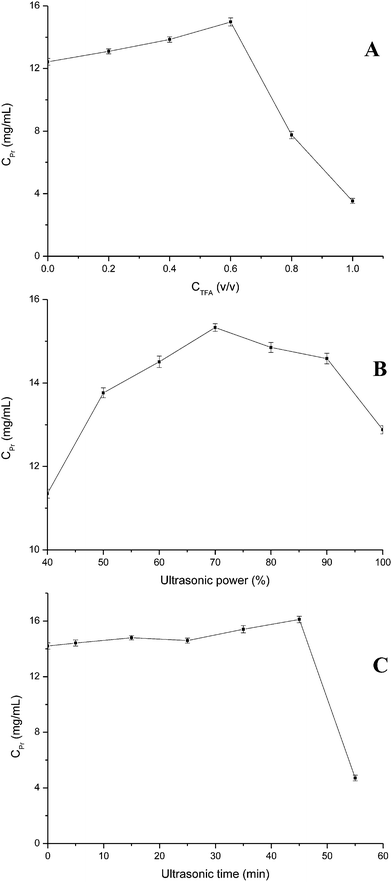 | ||
| Fig. 1 Effects of extraction parameters on protein extraction process. ((A) TFA concentration, %; (B) ultrasonic power, %; (C) ultrasonic time, min). | ||
Proteins in egg white contain a significant amount of polar and hydrophilic amino acid, such as glutamine/glutamic acid, lysine and arginine which contribute to favorable hydrophilic interactions between protein molecules and water via hydrogen bonds and electrostatic interactions.34 Hydrophilic interactions may also be partly responsible for the single-factor experiment results.
3.2. Response surface optimization
In order to systemically investigate the effects of extraction parameters on the concentration of protein extracted from egg white and their interactions, RSM employing BBD was used to optimize ultrasound-assisted extraction. The range and center point values of three independent variables were based on the results of the single-factor experiment. The results from 12 experimental runs and three central point runs (TFA concentration, 0.5%; ultrasonic power, 70% and ultrasonic time, 45 min) using BBD are presented in Table 1. By applying multiple regression analyses on the experimental data, the predicted response variables can be obtained by the following second-order polynomial equation: Y = 15.91 + 1.92A − 1.16B − 0.90C + 2.21AB + 0.53AC + 0.97BC − 3.70A2 − 2.32B2 − 0.85C2, where A, B, and C are in terms of coded factors of the test variables, namely TFA concentration, ultrasonic power and ultrasonic time.| Run | Independent variable | Response Y: C (Pr), mg mL−1 | ||
|---|---|---|---|---|
| A: C (TFA), % | B: t, min | C: W, % | ||
| 1 | 0.50 (0) | 60.00 (+1) | 40.00 (−1) | 11.92 |
| 2 | 0.50 (0) | 45.00 (0) | 70.00 (0) | 15.34 |
| 3 | 0.20 (−1) | 60.00 (+1) | 70.00 (0) | 3.91 |
| 4 | 0.80 (+1) | 45.00 (0) | 100.00 (+1) | 12.65 |
| 5 | 0.50 (0) | 30.00 (−1) | 40.00 (−1) | 15.41 |
| 6 | 0.50 (0) | 60.00 (+1) | 100.00 (+1) | 12.00 |
| 7 | 0.50 (0) | 45.00 (0) | 70.00 (0) | 16.17 |
| 8 | 0.20 (−1) | 45.00 (0) | 100.00 (+1) | 8.32 |
| 9 | 0.50 (0) | 30.00 (−1) | 100.00 (+1) | 11.62 |
| 10 | 0.20 (−1) | 30.00 (−1) | 70.00 (0) | 11.44 |
| 11 | 0.20 (−1) | 45.00 (0) | 40.00 (−1) | 11.13 |
| 12 | 0.80 (+1) | 45.00 (0) | 40.00 (−1) | 13.33 |
| 13 | 0.80 (+1) | 30.00 (−1) | 70.00 (0) | 11.44 |
| 14 | 0.80 (+1) | 60.00 (+1) | 70.00 (0) | 12.76 |
| 15 | 0.50 (0) | 45.00 (0) | 70.00 (0) | 16.24 |
A summary of analysis of variance (ANOVA) for the BBD experimental results is shown in Table 2. The smaller the P-values (p < 0.05) are, the bigger significance of the corresponding coefficient, which is implied that the response model was more suitable for reflecting the expected optimization. It can be also seen from Table 2 that F-value for the lack of fit was not significant (p > 0.05) relative to the pure error, confirming the validity of the model. In this case, A, B, C, AB, BC, A2 and B2 are significant model terms. So TFA concentration, ultrasonic power and ultrasonic time were important factors in the extraction process.
| Parameters | Sum of squares | df | Mean square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 138.06 | 9 | 15.34 | 32.33 | 0.0007 |
| A | 29.61 | 1 | 29.61 | 62.40 | 0.0005 |
| B | 10.84 | 1 | 10.84 | 22.84 | 0.0050 |
| C | 6.46 | 1 | 6.46 | 13.62 | 0.0141 |
| AB | 19.58 | 1 | 19.58 | 41.26 | 0.0014 |
| AC | 1.13 | 1 | 1.13 | 2.39 | 0.1830 |
| BC | 3.75 | 1 | 3.75 | 7.90 | 0.0375 |
| A2 | 50.60 | 1 | 50.60 | 106.62 | 0.0001 |
| B2 | 19.92 | 1 | 19.92 | 41.98 | 0.0013 |
| C2 | 2.69 | 1 | 2.69 | 5.67 | 0.0631 |
| Residual | 2.37 | 5 | 0.47 | — | — |
| Lack of fit | 1.87 | 3 | 0.62 | 2.49 | 0.2993 |
| Pure error | 0.50 | 2 | 0.25 | — | — |
The three-dimensional (3D) response surface and the contour plots (Fig. 2) were obtained using Design-Expert 7.0.0 software. They provide a means of visualizing the relationship between the responses and experimental levels of each variable and the type of interactions between the two test variables. As shown in Fig. 2, the mutual interaction between TFA concentration and ultrasonic power (Fig. 2B) was found to be more significant than the two others. It showed that the response was affected significantly by TFA concentration. It can be concluded that optimal extraction conditions of protein from egg white were following ranges of the examined variables: TFA concentration, 0.35–0.65% (v/v), ultrasonic power, 55–85% and ultrasonic time, 37.5–52.5 min. The optimum values of the test variables were TFA concentration, 0.53% (v/v); ultrasonic power, 49.41% and ultrasonic time, 39.89 min. Under these conditions, the maximum protein concentration was predicted as 16.5257 mg mL−1. Taking convenience into account, the optimum experimental parameters were modified as follows: TFA concentration, 0.53% (v/v), ultrasonic power, 50% and ultrasonic time, 40 min. To compare the predicted results with experimental values, rechecking was performed using modified optimal conditions. The result showed that experimental value (16.56 mg mL−1, n = 3) and predicted results (16.5257 mg mL−1) were not significant (p > 0.05).
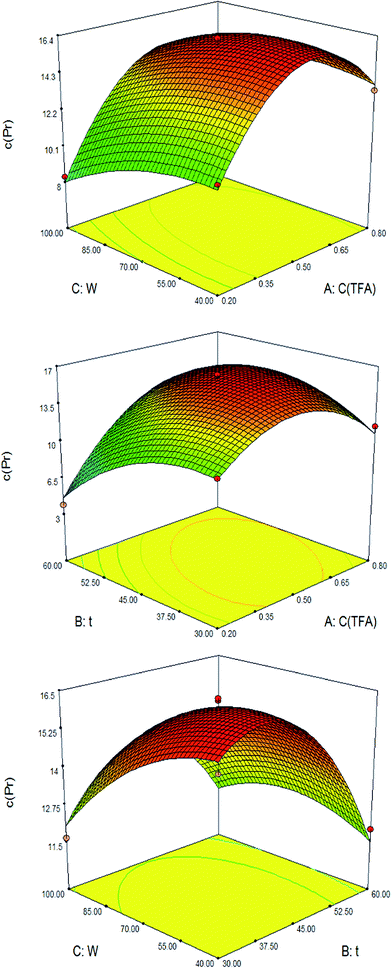 | ||
| Fig. 2 Response surface plots of the protein concentration affected by TFA concentration (v/v), ultrasonic time (min) and ultrasonic power (%). | ||
3.3. Effects of parameters in the process of hydrolysis
To establish an adequate system for enzymatic hydrolysis of the protein sample, the effects of tryptic aliquot, pH and incubation time were investigated. The experiment results were consistent with the reported condition of enzymatic hydrolysis.35 The extracted proteins were incubated in the presence of different aliquot (4, 6, 8, 10 μL) of 10 μg mL−1 trypsin and PBS (pH 7.0, 7.5, 8.0, 8.5) prior to mass spectrometric analysis. The effect of incubation time (10, 13, 16, 19, 22 h) was also investigated (Table 3). The results show that the number of peptides increased from 7 to 17 with the trypsin aliquot increasing from 4 μL to 8 μL and decreased to 9 with the trypsin aliquot increasing to 10 μL. 8 μL of 10 μg mL−1 trypsin was selected in the process of hydrolysis. A few peptides generated as result of partly enzymatic action in the presence of insufficient trypin. The trypsin may be degraded by itself when excessive trypin was added, which affects the identification of protein. The number of peptides decreased from 19 to 9 with the pH increasing from 7.0 to 8.0 and changed slightly with the pH increasing from 8.0 to 8.5. This showed that the trypin has higher activity with a pH value of 7.0 in the tryptic hydrolysis of the proteins. Prolonged incubation time from 10 h to 16 h, the number of peptides increased from 9 to 14 and then began to change slightly, so the suitable incubation time was selected at 16 h.| Parameters | Number of peptides | ||||
|---|---|---|---|---|---|
| V (trypsin), μL | pH | t, h | No. (V) | No. (pH) | No. (t) |
| 4 | 7.0 | 10 | 7 | 19 | 9 |
| 6 | 7.5 | 13 | 14 | 7 | 8 |
| 8 | 8.0 | 16 | 17 | 9 | 14 |
| 10 | 8.5 | 19 | 9 | 9 | 14 |
| — | — | 22 | — | — | 12 |
3.4. Identification of protein
Since all the biological liquids previously described in reality bathe a number of tissues in the human organism, one would expect to find plenty of proteins, especially novel proteins. PMF approach29 for protein identification implies that each protein subjected to tryptic hydrolysis give a unique set of peptides which represent fingerprint of the protein. Fig. 3 displays the mass spectra of the hydrolyzed protein extracted from the egg white. The different proteins identified in the free-range chicken egg white and barn-raised chicken egg white were summarized in Tables 4 and 5. 23 proteins for free-range chicken egg white and 29 proteins for barn-raised chicken egg white were identified respectively. Among them, 16 proteins were identified in both two egg white varieties including well-known proteins such as ovalbumin, ovostransferrin, lysozyme C and ovostatin, which were already identified by D'Ambrosio et al.11 Collagen peptides were identified for the first time. 6 collagen peptides were detected in free-range chicken egg white, namely collagen alpha-3 (VI) chain, collagen alpha-1 (XII) chain, collagen alpha-2 (I) chain (Fragments), 72 kDa type IV collagenase, collagen alpha-3 (IX) chain and collagen alpha-1(VIII) chain. 9 collagen peptides were detected in barn-raised chicken egg white, namely collagen alpha-3 (VI) chain, collagen alpha-1 (XII) chain, collagen alpha-2 (I) chain (Fragments), 72 kDa type IV collagenase, collagen alpha-1 (III) chain (Fragments), collagen alpha-2 (VI) chain, collagen alpha-2 (IX) chain, collagen alpha-1 (I) chain and collagen alpha-1 (XIV) chain.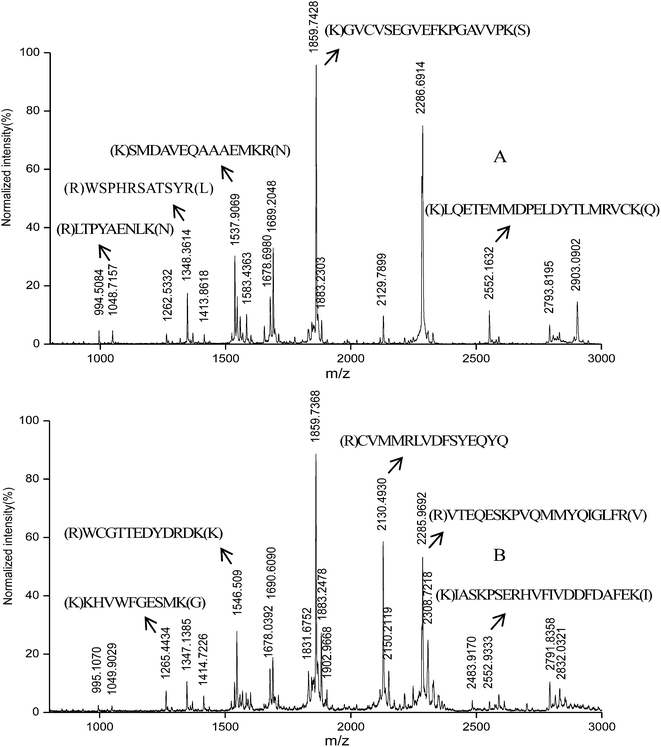 | ||
| Fig. 3 MALDI-TOF-MS spectrum of the hydrolyzed protein extracted from the egg white in free-range chicken eggs (A) and barn-raised chicken eggs (B). | ||
| Accession no. | Protein name | Mowse score | #pep #mat %mat | Cov % | MS-digest index no. | Peptide MW m/z | Position | M. cut | Sequence | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Exp. | Theor. | Start | End | ||||||||
| a Asterisk indicates proteins detected in both two egg whites. Proteins detected only in free-range chicken egg white are indicated with (#) sign. Proteins detected for the first time in free-range chicken egg white are indicated with section mark (§). | |||||||||||
| P49702 | ADP-ribosylation factor 5* | 217 | 5/4/18 | 32.8 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 411 411 |
1678.6980 | 1678.9325 | 128 | 142 | 0 | (K)QDMPNAMVVSELTDK(L) |
| P08250 | Apolipoprotein A-I* | 226 | 5/5/23 | 18.2 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 522 522 |
1048.7157 | 1049.2193 | 241 | 249 | 0 | (R)LTPYAENLK(N) |
| Q90593 | 78 kDa glucose-regulated protein# | 36.4 | 5/4/18 | 8.1 | 155![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 514 514 |
1537.9069 | 1537.8265 | 137 | 150 | 0 | (K)TFAPEEISAMVLTK(M) |
| P15505 | Glycinedehydrogenase# | 551 | 9/7/32 | 15.2 | 142![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 275 275 |
1413.8618 | 1413.6381 | 15 | 28 | 1 | (R)GAPRHLRPAAGGPR(R) |
| Q02391 | Golgi apparatus protein 1# | 59.2 | 10/7/32 | 10.1 | 157![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 307 307 |
2552.1632 | 2551.9534 | 829 | 848 | 1 | (K)LQETEMMDPELDYTLMRVC(Carbamidomethyl)K(Q) |
| P09987 | Histone H1* | 298 | 4/4/18 | 26.6 | 159![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 972 972 |
1654.8826 | 1654.8469 | 1 | 18 | 0 | (−)MSETAPVAAPAVSAPGAK(A) |
| P00698 | Lysozyme C# | 964 | 5/5/23 | 36.7 | 217![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 798 798 |
2129.7899 | 2129.7823 | 1 | 19 | 1 | (−)MRSLLILVLC(Carbamidomethyl)FLPLAALGK(V) |
| Q8AXY6 | Muscle, skeletal receptor tyrosine protein kinase# | 217 | 6/5/23 | 10.6 | 247![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 982 982 |
1654.8826 | 1654.9153 | 297 | 311 | 1 | (K)AAATISVSEWSKLYK(G) |
| P01012 | Ovalbumin# | 1942 | 8/5/23 | 29.5 | 277![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 728 728 |
2326.6172 | 2326.7314 | 1 | 20 | 1 | (−)MGSIGAASMEFC(Carbamidomethyl)FDVFKELK(V) |
| P01014 | Ovalbumin-related protein Y# | 299 | 4/4/18 | 11.1 | 277![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 727 727 |
994.5084 | 994.2516 | 278 | 285 | 1 | (K)SMKVYLPR(M) |
| 1413.8618 | 1413.6289 | 373 | 384 | 0 | (R)YNPTNAILFFGR(Y) | ||||||
| Q98UI9 | Mucin-5B# | 3881 | 8/6/27 | 6.1 | 243![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 903 903 |
1859.7428 | 1860.1960 | 1870 | 1887 | 0 | (K)GVC(Carbamidomethyl)VSEGVEFKPGAVVPK(S) |
| P20740 | Ovostatin# | 80.0 | 9/8/36 | 9.1 | 277![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 762 762 |
994.5084 | 994.2486 | 1382 | 1390 | 0 | (K)MLSGFVPVK(S) |
| 1413.8618 | 1413.6646 | 921 | 931 | 0 | (R)EETQNFLIC(Carbamidomethyl)MK(D) | ||||||
| 1537.9069 | 1537.8509 | 421 | 433 | 1 | (K)IFDPELSLKALYK(T) | ||||||
| P02789 | Ovotransferrin# | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 938 938 |
8/8/36 | 16.5 | 463![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 788 788 |
994.5084 | 994.1017 | 633 | 641 | 1 | (K)RFGVNGSEK(S) |
| Q91348 | 6-Phosphofructo-2-kinase/fructose-2,6-bisphosphatase# | 30.4 | 5/5/23 | 15.3 | 120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 743 743 |
1583.4363 | 1583.7700 | 361 | 373 | 1 | (R)YPKGESYEDLVQR(L) |
| P26446 | Poly[ADP-ribose] polymerase 1* | 238 | 9/8/36 | 12.7 | 282![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 338 338 |
1883.2303 | 1883.8336 | 282 | 298 | 0 | (R)VADGMAFGALLPC(Carbamidomethyl)EEC (Carbamidomethyl)K(G) |
| 2129.7899 | 2130.4211 | 484 | 502 | 1 | (K)TEHQEVAVDGKC(Carbamidomethyl)SKPANMK(S) | ||||||
| P19121 | Serum albumin* | 96.9 | 8/7/32 | 18.2 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 314 314 |
994.5084 | 994.2296 | 434 | 441 | 1 | (K)SILIRYTK(K) |
| 1558.9205 | 1558.8455 | 443 | 456 | 0 | (K)MPQVPTDLLLETGK(K) | ||||||
| P10039 | Tenascin# | 1689 | 9/7/32 | 7.9 | 452![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 759 759 |
1262.5332 | 1262.5074 | 195 | 204 | 1 | (R)NC(Carbamidomethyl)LNRGLC(Carbamidomethyl)VR(G) |
| 1262.5332 | 1262.4135 | 858 | 868 | 0 | (K)EVFVTDLDAPR(N) | ||||||
| P15989 | Collagen alpha-3(VI) chain#,§ | 2.30 × 107 | 30/18/82 | 14.2 | 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 803 803 |
1537.9069 | 1537.7645 | 560 | 573 | 1 | (K)SMDAVEQAAAEMKR(N) |
| 1545.6119 | 1545.7431 | 3030 | 3043 | 1 | (K)SQPKVTYTGTFSTK(T) | ||||||
| 2326.6172 | 2326.6628 | 800 | 818 | 0 | (K)MVYFMDDFSDLTTLPQELK(K) | ||||||
| P13944 | Collagen alpha-1(XII) chain#,§ | 1.30 × 106 | 25/13/59 | 11.8 | 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 538 538 |
1348.3614 | 1348.4725 | 2228 | 2238 | 1 | (R)WSPHRSATSYR(L) |
| 1545.6119 | 1545.7027 | 766 | 779 | 1 | (R)QVTVSANERSTTLR(N) | ||||||
| P02467 | Collagen alpha-2(I) chain#,§ | 206 | 6/4/18 | 8.5 | 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 671 671 |
1654.8826 | 1654.8999 | 412 | 430 | 1 | (R)AGVMGPAGNRGASGPVGAK(G) |
| Q90611 | 72 kDa type IV collagenase#,§ | 165 | 7/6/27 | 11.2 | 232![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 153 153 |
1545.6119 | 1545.6274 | 313 | 324 | 1 | (R)WC(Carbamidomethyl)GTTEDYDRDK(K) |
| P32017 | Collagen alpha-3(IX) chain*,§ | 66.9 | 4/4/18 | 10.2 | 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 896 896 |
2326.6172 | 2325.6024 | 203 | 227 | 1 | (K)EGEKGSPGPPGPPGIPGSVGLQGPR(G) |
| Q7LZR2 | Collagen alpha-1(VIII) chain*,§ | 40.9 | 4/4/18 | 8.6 | 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 870 870 |
1348.3614 | 1347.5229 | 414 | 428 | 0 | (K)GEGGIVGPQGPPGPK(G) |
| Accession no. | Protein name | Mowse score | #pep #mat %mat | Cov. % | MS-digest index no. | Peptide MW m/z | Position | M. cut | Sequence | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Exp. | Theor. | Start | End | ||||||||
| a Asterisk indicates proteins detected in both two egg whites. Proteins detected only in barn-raised chicken egg white are indicated with (#) sign. Proteins detected for the first time in barn-raised chicken egg white are indicated with section mark (§). | |||||||||||
| O57579 | Aminopeptidase N* | 4315 | 6/6/18 | 10.2 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 844 844 |
1524.7059 | 1524.7304 | 669 | 682 | 0 | (R)AHNVNVTLALNTTR(F) |
| Q90839 | Dickkopf-related protein 3* | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 274 274 |
6/6/18 | 24.6 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 229 229 |
1535.7096 | 1535.6363 | 59 | 72 | 0 | (R)NAVQEMEAEEEGAK(K) |
| Q90593 | 78 kDa glucose-regulated protein# | 327![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 721 721 |
10/9/26 | 18.4 | 155![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 514 514 |
1535.7096 | 1535.7539 | 122 | 136 | 0 | (K)AKPHIQVDVGGGQTK(T) |
| 1567.8431 | 1567.7493 | 59 | 72 | 0 | (R)ITPSYVAFTPEGER(L) | ||||||
| 1678.0392 | 1678.8069 | 80 | 94 | 0 | (K)NQLTSNPENTVFDAK(R) | ||||||
| 2150.2119 | 2150.3176 | 305 | 322 | 0 | (R)IEIESFFEGEDFSETLTR(A) | ||||||
| P15505 | Glycine dehydrogenase# | 5832 | 8/7/21 | 12.6 | 142![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 275 275 |
1559.6078 | 1559.7738 | 633 | 648 | 0 | (R)SAHGTNPASAQMAGMK(I) |
| Q02391 | Golgi apparatus protein 1# | 537 | 15/12/35 | 15.1 | 157![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 307 307 |
1599.6078 | 1599.8790 | 324 | 337 | 1 | (K)LIAQDYKVSYSLAK(S) |
| P01875 | Ig mu chain C region* | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 940 940 |
5/5/15 | 13.5 | 183![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 863 863 |
1535.7096 | 1335.7168 | 141 | 150 | 1 | (R)RRPTEVTWYK(N) |
| P00698 | Lysozyme C# | 610 | 5/4/12 | 36.1 | 217![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 798 798 |
2171.7799 | 2171.8199 | 1 | 19 | 1 | (−)MRSLLILVLC(Carbamidomethyl)FLPLAALGK(V) |
| Q8AXY6 | Muscle, skeletal receptor tyrosine protein kinase# | 3279 | 8/7/21 | 14.1 | 247![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 982 982 |
2248.0482 | 2248.0339 | 827 | 845 | 1 | (R)NMYSADYYKANENDAIPIR(W) |
| Q5ZJH2 | Nicalin* | 3977 | 7/6/18 | 15.5 | 254![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 388 388 |
1414.7226 | 1414.6605 | 316 | 328 | 0 | (R)GNSLHLHVSKPPK(E) |
| 2130.4930 | 2130.4835 | 83 | 98 | 1 | (R)C(Carbamidomethyl)VMMRLVDFSYEQYQK(A) | ||||||
| P01012 | Ovalbumin# | 180![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 084 084 |
11/8/24 | 29.8 | 277![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 728 728 |
2285.9692 | 2285.7061 | 201 | 219 | 0 | (R)VTEQESKPVQMMYQIGLFR(V) |
| P01014 | Ovalbumin-related protein Y# | 895 | 4/4/12 | 14.4 | 277![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 727 727 |
995.1070 | 994.2516 | 278 | 285 | 1 | (K)SMKVYLPR(M) |
| Q98UI9 | Mucin-5B# | 1.46 × 107 | 16/14/41 | 11.3 | 243![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 903 903 |
1678.0392 | 1677.8629 | 941 | 955 | 1 | (R)IQEIATDPGAEKNYK(V) |
| 2150.2119 | 2150.4951 | 85 | 103 | 0 | (K)NSHLIYFTVTTDGVILEVK(E) | ||||||
| P20740 | Ovostatin# | 514![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 326 326 |
16/11/32 | 13.8 | 277![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 762 762 |
1711.7647 | 1711.9209 | 762 | 776 | 0 | (K)ASVSYTIPDTITEWK(A) |
| P02789 | Ovotransferrin# | 2.85 × 107 | 10/10/29 | 25.2 | 463![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 788 788 |
1535.7096 | 1535.8002 | 141 | 154 | 0 | (R)SAGWNIPIGTLLHR(G) |
| 1678.0392 | 1677.9748 | 540 | 553 | 0 | (K)YFGYTGALRC(Carbamidomethyl)LVEK(G) | ||||||
| Q91348 | 6-phosphofructo-2-Kinase/fructose-2,6-bisphosphatase# | 2220 | 9/9/26 | 28.3 | 120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 743 743 |
1567.8431 | 1567.8438 | 1 | 16 | 0 | (−)MAAVASGQLTQNPLQK(V) |
| Q05199 | Pro-neuregulin-1, membrane-bound isoform* | 1464 | 6/6/18 | 16.9 | 264![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 094 094 |
1535.7096 | 1535.8139 | 147 | 159 | 0 | (K)AFC(Carbamidomethyl)VNGGEC(Carbamidomethyl) YMVK(D) |
| 2308.7218 | 2308.5198 | 118 | 140 | 0 | (K)ASVIITDTNATSTSTTGTSHLTK(C) | ||||||
| Q8JGM4 | Sulfhydryl oxidase 1* | 827 | 4/4/12 | 8.7 | 322![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 688 688 |
1265.4434 | 1265.4637 | 147 | 158 | 0 | (R)IAHPTATVADLR(R) |
| P10039 | Tenascin# | 431![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 586 586 |
13/12/35 | 11.4 | 452![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 759 759 |
1678.0392 | 1677.8659 | 872 | 885 | 1 | (K)RVSQTDNSITLEWK(N) |
| P41366 | Vitelline membrane outer layer protein 1* | 4167 | 5/5/15 | 31.7 | 489![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 488 488 |
2171.7799 | 2171.6473 | 3 | 21 | 0 | (K)VLTPAALILLFFFYTVDAR(T) |
| P47990 | Xanthine dehydrogenase/oxidase* | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 977 977 |
14/11/32 | 18.0 | 493![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 835 835 |
2552.9333 | 2552.9467 | 45 | 67 | 1 | (K)LGC(Carbamidomethyl)GEGGC(Carbamidomethyl) GAC(Carbamidomethyl)TVMISKYDPFQK(K) |
| P15989 | Collagen alpha-3(VI) chain#,§ | 2.62 × 1010 | 37/25/74 | 18.1 | 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 803 803 |
1524.7059 | 1524.8177 | 705 | 717 | 1 | (K)SDIIQRLGQLRPK(G) |
| 2328.4408 | 2328.6027 | 2670 | 2690 | 0 | (R)VAVLQQAPYDHETNSSFPPVK(T) | ||||||
| P13944 | Collagen alpha-1(XII) chain#,§ | 1.42 × 109 | 40/22/65 | 19.3 | 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 538 538 |
2552.9333 | 2552.8264 | 2475 | 2495 | 1 | (K)IASKPSERHVFIVDDFDAFEK(I) |
| P12105 | Collagen alpha-1(III) chain*,§ | 108![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 409 409 |
13/11/32 | 19.5 | 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 727 727 |
1265.4434 | 1265.4854 | 1120 | 1129 | 1 | (K)KHVWFGESMK(G) |
| 1883.2478 | 1883.1492 | 572 | 591 | 1 | (R)GQPGVMGFPGPKGNEGAPGK(N) | ||||||
| P15988 | Collagen alpha-2(VI) chain*,§ | 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 993 993 |
11/10/29 | 17.4 | 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
1369.3730 | 1369.4858 | 475 | 489 | 0 | (R)GPTGAVGEPGNIGSR(G) |
| 1524.7059 | 1524.6808 | 676 | 688 | 1 | (K)LDDERINSLSSFK(E) | ||||||
| P02467 | Collagen alpha-2(I) chain#,§ | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 288 288 |
7/7/21 | 10.0 | 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 671 671 |
1414.7226 | 1414.6112 | 1285 | 1297 | 0 | (K)AVILQGSNDVELR(A) |
| 1678.0392 | 1677.8252 | 180 | 197 | 0 | (R)GHNGLDGLTGQPGAPGTK(G) | ||||||
| P12108 | Collagen alpha-2(IX) chain*,§ | 6462 | 10/8/24 | 13.3 | 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 893 893 |
1265.4434 | 1265.3735 | 265 | 277 | 1 | (R)EGPKGPPGDPGEK(G) |
| P32018 | Collagen alpha-1(XIV) chain*,§ | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5424 5424 |
16/13/38 | 12.1 | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 688 688 |
1524.7059 | 1524.7961 | 497 | 511 | 1 | (R)GLLGERGVPGMPGQR(G) |
| 1567.8431 | 1567.8316 | 530 | 543 | 0 | (K)MMQEQLAEVAVSAK(R) | ||||||
| Q90611 | 72 kDa type IV collagenase#,§ | 1434 | 10/7/21 | 19.2 | 232![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 153 153 |
1546.5090 | 1546.6218 | 313 | 324 | 1 | (R)WC(Carbamidomethyl)GTTEDYDRDK(K) |
| 1678.0392 | 1677.8819 | 637 | 649 | 1 | (K)DQYYLQMEDKSLK(I) | ||||||
| P02457 | Collagen alpha-1(I) chain*,§ | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 638 638 |
12/9/26 | 14.0 | 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 660 660 |
1265.4434 | 1265.3521 | 1086 | 1097 | 1 | (K)GETGEQGDRGMK(G) |
| 1559.6078 | 1559.7731 | 753 | 770 | 0 | (R)GLTGPIGPPGPAGAPGDK(G) | ||||||
| 1711.7647 | 1711.8182 | 276 | 293 | 0 | (K)GEPGSPGENGAPGQMGPR(G) | ||||||
The main nutritional characteristics of the two chicken varieties may also be partly responsible for their deferent proteins in egg white. These differences were most probably caused by differences in the intake of feedstuffs of the free-range and barn-raised chicken. The food of free-range chicken can come from a variety of different sources, including their feed, worms, insects, grass, herbs and soil. Barn-raised chickens, however, are fed on a mixture of known feedstuffs prepared from corn, soybean meal, animal-products and synthetic additive.36,37 Thus, the protein from the animal-products in the mixture of known feedstuffs, such as protein feeds, which are the food products resulting from the hydrolysis of animal tissues such as bones and skin, could be found in the egg white of the barn-raised chickens. Compared to free-range chicken egg white, more big molecular mass proteins exist in the egg white from barn-raised chicken when the feedstuff which is rich in proteins were part absorbed by chickens and the unabsorbed big molecular mass proteins may be accumulated in its tissues. This reason may lead to more species of proteins in egg white from barn-raised chicken than from free-range chicken.
Some other proteins reported previously may be hidden and failed to detect in this current study probably because the fractionation techniques were not adopted.
4. Conclusion
In the present report, PMF approach of identification of protein in egg white were established and successfully applied to identify the proteins in egg white from free-range and barn-raised chicken by MALDI-TOF-MS. Extraction process of protein were optimized using RSM method with BBD. Besides that, enzymatic hydrolysis conditions were optimized, especially that the effect of pH on tryptic hydrolysis of proteins was successfully investigated via dialyzing against PBS of different pH. The experimental results show that the species of proteins in barn-raised chicken egg white was more than in free-range chicken egg white. The collagen peptides were identified in both two egg whites for the first time. The knowledge of the protein composition of egg white may contribute to the food industry and health care. Further detailed study will be necessary to characterize these novel proteins and to determine their possible function.References
- Y. Mine, Trends Food Sci. Technol., 1995, 6, 225–232 CrossRef CAS.
- J. Kovacs-Nolan, M. Phillips and Y. Mine, J. Agric. Food Chem., 2005, 53, 8421–8431 CrossRef CAS PubMed.
- L. Stevens, Comp. Biochem. Physiol., Part B: Biochem. Mol. Biol., 1991, 100, 1–9 CrossRef CAS.
- L. J. Zimmerman, G. R. Wernke, R. M. Caprioli and D. C. Liebler, J. Proteome Res., 2005, 4, 1672–1680 CrossRef CAS PubMed.
- Y. Hioki, R. Tanimura, S. Iwamoto and K. Tanaka, Anal. Chem., 2014, 86, 2549–2558 CrossRef CAS PubMed.
- M. T. M. Blaze, B. Aydin, R. P. Carlson and L. Hanley, Analyst, 2012, 137, 5018–5025 RSC.
- N. Bergman and J. Bergquist, Analyst, 2014, 1–16 Search PubMed.
- C. Guérin-Dubiard, M. Pasco, D. Mollé, C. Désert, T. Croguennec and F. Nau, J. Agric. Food Chem., 2006, 54, 3901–3910 CrossRef PubMed.
- V. Raikos, R. Hansen, L. Campbell and S. R. Euston, Food Chem., 2006, 99, 702–710 CrossRef CAS PubMed.
- K. Mann, Proteomics, 2007, 7, 3558–3568 CrossRef CAS PubMed.
- C. D. Ambrosio, S. Arena, A. Scaloni, L. Guerrier, E. Boschetti, M. E. Mendieta, A. Citterio and P. G. Righetti, J. Proteome Res., 2008, 7, 3461–3474 CrossRef PubMed.
- D. A. Omana, Y. Liang, N. N. V. Kav and J. Wu, Proteomics, 2011, 11, 144–153 CrossRef CAS PubMed.
- F. G. Silversides and K. Budgell, Poult. Sci., 2004, 83, 1619–1623 CrossRef CAS PubMed.
- J. Schlatterer and D. E. Breithaupt, J. Agric. Food Chem., 2006, 54, 2267–2273 CrossRef CAS PubMed.
- A. Hidalgo, M. Rossi, F. Clerici and S. Ratti, Food Chem., 2008, 106, 1031–1038 CrossRef CAS PubMed.
- M. Roszko, K. Szymczyk and R. Jędrzejczak, Sci. Total Environ., 2014, 487, 279–289 CrossRef CAS PubMed.
- H. C. Lee, A. K. Htoon and J. L. Paterson, Food Chem., 2007, 102, 551–559 CrossRef CAS PubMed.
- C. L. Ye and C. J. Jiang, Carbohydr. Polym., 2011, 84, 495–502 CrossRef CAS PubMed.
- E. Dorta, M. Gloria Lobo and M. Gonzalez, Food Bioprocess Technol., 2013, 6, 1067–1081 CrossRef CAS.
- B. Du, F. M. Zhu and B. J. Xu, J. Cereal Sci., 2014, 59, 95–100 CrossRef CAS PubMed.
- M. M. Bradford, Anal. Biochem., 1976, 72, 248–254 CrossRef CAS.
- N. Carlsson, A. Borde, S. Wölfel, B. Åkerman and A. Larsson, Anal. Biochem., 2011, 411, 116–121 CrossRef CAS PubMed.
- Q. Z. Jin, X. Q. Zou, L. Shan, X. G. Wang and A. Y. Qiu, J. Agric. Food Chem., 2009, 58, 155–160 CrossRef PubMed.
- R. Minjares-Fuentes, A. Femenia, M. C. Garau, L. A. Meza-Velazquez, S. Simal and C. Rossello, Carbohydr. Polym., 2014, 106, 179–189 CrossRef CAS PubMed.
- Y. Liu, G. L. Gong, J. Zhang, S. Y. Jia, F. Li, Y. Y. Wang and S. H. Wu, Carbohydr. Polym., 2014, 110, 278–284 CrossRef CAS PubMed.
- C. Tokarski, E. Martin, C. Rolando and C. Cren-Olivé, Anal. Chem., 2006, 78, 1494–1502 CrossRef CAS PubMed.
- Y. Y. Li, P. Hao, S. L. Zhang and Y. X. Li, Mol. Cell. Proteomics, 2011, 10, M110–M5785 Search PubMed.
- R. Hynek, S. Kuckova, J. Hradilova and M. Kodicek, Rapid Commun. Mass Spectrom., 2004, 18, 1896–1900 CrossRef CAS PubMed.
- I. D. van der Werf, C. D. Calvano, F. Palmisano and L. Sabbatini, Anal. Chim. Acta, 2012, 718, 1–10 CrossRef CAS PubMed.
- G. Gautier and M. P. Colombini, Talanta, 2007, 73, 95–102 CrossRef CAS PubMed.
- H. T. Yan, J. J. An, T. Zhou and Y. H. Li, Chin. Sci. Bull., 2013, 58, 2932–2937 CrossRef CAS.
- C. Guérin-Dubiard, M. Pasco, A. Hietanen, A. Q. Bosque, F. Nau and T. Croguennec, J. Chromatogr. A, 2005, 1090, 58–67 CrossRef PubMed.
- J. Wang, Y. Liang, D. A. Omana, N. N. V. Kav and J. P. Wu, J. Agric. Food Chem., 2012, 60, 272–282 CrossRef CAS PubMed.
- M. Jarpa-Parra, F. Bamdad, Y. Wang, Z. Tian, F. Temelli, J. Han and L. Chen, LWT--Food Sci. Technol., 2014, 57, 461–469 CrossRef CAS PubMed.
- A. Shevchenko, H. Tomas, J. Havliš, J. V. Olsen and M. Mann, Nat. Protoc., 2007, 1, 2856–2860 CrossRef PubMed.
- L. D. Coletta, A. L. Pereira, A. A. D. Coelho, V. J. M. Savino, J. F. M. Menten, E. Correr, L. C. Franca and L. A. Martinelli, Food Chem., 2012, 131, 155–160 CrossRef CAS PubMed.
- E. N. Sossidou, A. Dal Bosco, H. A. Elson and C. M. G. A. Fontes, World's Poult. Sci. J., 2011, 67, 47–58 CrossRef.
| This journal is © The Royal Society of Chemistry 2014 |
