Biological co-production of ethanol and biodiesel from wheat straw: a case of dilute acid pretreatment†
Abstract
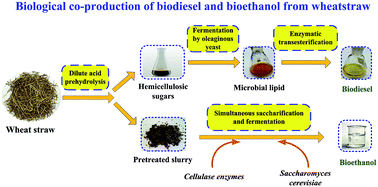
A process for co-production of ethanol and biodiesel from wheat straw was proposed. Dilute acid pre-hydrolysis of hemicellulose followed by enzymatic hydrolysis of cellulose were optimized to maximize recovery of total sugars. It was found that xylose yield obtained by super-dilute acid (0–0.1%) pretreatment under the experimental conditions was too low. By using moderate conditions (140–160 °C) with higher sulfuric acid concentration (0.3–0.6%), xylose recovery could be greatly increased to 60–70%. The relatively optimum conditions for dilute acid pretreatment were 0.5% H2SO4 at 140 °C for 1 h. 15.1 g L−1 ethanol with approximately 58% of theoretical yield obtained by SSF of the pretreated solid. The hydrolyzate was directly converted to microbial lipid using a mutagenized Rhodosporidium toruloides. The extracted lipid was well converted to biodiesel with 90% conversion ratio under the catalysis of immobilized lipase. Mass balance showed that 0.80 g biodiesel and 10.1 g ethanol were produced from 100 g of wheat straw. This work thus can provide a novel idea for biological production of biofuels from lignocellulosic biomass.

 Please wait while we load your content...
Please wait while we load your content...