A 1H NMR-based metabonomic investigation of time-dependent metabolic trajectories in a high salt-induced hypertension rat model
Linlin Wangab,
Lingyun Zhenga,
Ren Luoc,
Xiaoshan Zhaoc,
Zhihui Hanab,
Yaling Wangab and
Yongxia Yang*a
aSchool of Basic Courses, Guangdong Pharmaceutical University, Guangzhou, 510006, P. R. China. E-mail: sheepma@163.com; Fax: +86-20-39352197; Tel: +86-20-39352197
bSchool of Traditional Chinese Medicine, Guangdong Pharmaceutical University, Guangzhou, 510006, P. R. China
cDepartment of Traditional Chinese Medicine, Southern Medical University, Guangzhou, 510515, P. R. China
First published on 23rd September 2014
Abstract
High salt-induced hypertension (HT) is an increasingly relevant health issue. However, the molecular mechanism of the metabolic transformation in HT development remains largely unknown. Features of the time-dependent metabolic transfer during HT onset and development should describe crucial aspects of HT phenotypes and may allow early prevention. To investigate the progression of HT and identify potential biomarkers, the metabolic profiles of urine, plasma, and fecal extracts of HT rats fed a high salt diet were investigated using a 1H nuclear magnetic resonance (NMR)-based metabonomics approach. In this study, the profiles at the 4th and 8th weeks for urine and fecal extracts could be classified, which revealed progression axes from normal status to HT status. Changes in succinate, α-ketoglutaric acid (α-KG), citrate, creatine and creatinine, choline, phosphocholine (PC) and glycerophosphocholine (GPC), trimethylamine-N-oxide and betaine, taurine and hippurate in urine, in conjunction with gut flora disturbance in feces were observed during the initial stage of HT (6th week). During the severe HT period (8th week), these metabolic changes became more pronounced, and the metabolic disturbance in plasma lipid and choline indicated a possible increased risk of cardiovascular diseases. Thus, an increase in dietary salt can induce a series of metabolic changes, and 1H NMR-based metabonomics offers a non-invasive means to elucidate the progression of HT induced by this dietary pattern.
Introduction
Hypertension (HT) is one of the most common chronic diseases and it affects about 30% of adults worldwide. HT is an important risk factor in cardiovascular, cerebrovascular and renal diseases, and is a leading risk factor of human mortality.1,2 HT is a multifactorial trait resulting from environmental and genetic factors. Excessive dietary salt intake is the most common environmental factor that leads to the pathogenesis of hypertension.3,4 Many epidemiological and genetic studies suggest that high salt intake is almost always associated with an increase in blood pressure in normotensive and hypertensive humans.5,6 The recommended minimal daily salt intake necessary for essential physiological functions is 1.5 g per day. The World Health Organization recommends that daily salt intake should be in a range of 3–5 g, and more than 6 g over a long time will increase the incidence of HT. However, the salt content of the traditional Chinese diet far exceeds this. By the end of 2012, 266 million people in China were thought to have HT, and the number of people suffering from HT in China is growing by 10 million annually.Reports show that HT is associated with dyslipidemia and diabetes mellitus (DM).7,8 About 75% of individuals with type 2 diabetes mellitus also have HT,9 and the incidence of both HT and DM are increasing every year. González et al.10 reported that a high salt intake induced a significant increase in insulin resistance index and a decrease in HDL, which showed that high salt intake caused lipid metabolism disturbances. Baudrand et al.7 reported that a high salt diet was associated with dyslipidaemia and hypoadiponectinaemia, and predicted metabolic syndrome (MS) status. Studies have shown that administration of a high salt diet to normal rats induced hypertension and MS.11–13 Elevated salt intake can also increase left ventricular mass in humans14 and animals,15 and further increase the risk of cardiovascular disease. Consequently, it is vital to examine the effects of excessive dietary salt intake on the metabonome systematically and the time-dependent metabolic trajectories induced by this dietary pattern, in order to explore the pathogenesis of HT from the perspective of metabolic regulation.
Metabonomics offers an alternative method for monitoring the biochemical changes induced by endogenous and exogenous factors.16,17 High-resolution 1H NMR spectroscopy of biofluids and tissues is an effective nondestructive method for probing the metabolic responses in a whole organism.18,19 Together with pattern recognition (PR) data analysis techniques, such as principal component analysis (PCA) and orthogonal partial least squares projection on discriminant analysis (OPLS-DA), NMR-based metabonomics has been extensively used to detect the metabolic changes in many metabolic diseases, such as diabetes mellitus,20 obesity21 and cardiovascular diseases.22,23 Seijo and co-workers diagnosed idiopathic portal hypertension (IPH) based on metabolomic profiles, and showed that metabolomic analysis could be a valuable tool for the noninvasive diagnosis of IPH.24 However, a systematic metabonomic study for HT induced by a high salt diet has not been reported.
In this work, we established the HT model induced by feeding rats an 8% salt diet, similar to the modeling method previously reported.25 An 1H NMR-based metabonomic approach was used in conjunction with multivariate data analysis to study the time-dependent metabolic changes in urine, plasma, and feces in the HT model. Blood pressure (BP), clinical biochemistry, and histopathology were also determined. The purpose of this study was to explore the systematic biochemical trajectories and progression of HT caused by this dietary pattern.
Experimental
Animal handling
Twenty male Wistar rats (weighing 200 ± 10 g) were purchased from Medical Laboratory Animal Center of Sun Yat-Sen University. The animals were housed in a well-ventilated animal experimental laboratory with a 12 h light/dark cycle, a constant temperature of 25 ± 1 °C, and a relative humidity of 50 ± 10%. This study was reviewed and approved by the Ethics Committee of Guangdong Pharmaceutical University. Rats were allowed free access to food and pure water. After acclimatization for one week, the rats were randomly divided into two groups with ten rats in each group, i.e. a control group and a high salt-fed group. The control rats were administered a normal diet (0.5% NaCl) for 8 weeks. The model rats were consecutively fed a high salt diet (8% NaCl) during the whole study for 8 weeks. This HT modeling method was the same as that reported by Gu et al.25Body weight measurement, blood glucose and blood pressure
At the end of the 4th, 6th and 8th weeks, the body weight of each rat was measured. Fasting blood glucose was determined colorimetrically by using a Randox reagent kit, which uses the glucose oxidase method called the Trinder reaction. Distilled water, glucose standard solution, or the blood sample (10 μL each) were added to three separate tubes, and reagent containing the enzyme and phenol (1000 μL) was added to each tube. The tubes were mixed well and incubated at 37 °C for 10 min. We measured the absorption values for each tube at a wavelength of 505 nm and calculated the blood glucose value. Blood pressure was measured by using the model RBP-1 animal BP meter. The independent sample t test was conducted to compare the biochemical data from the HT model rats and the control rats.Sample collection
During the experiment, blood samples were collected at the end of the 4th, 6th and 8th weeks from the orbital venous plexus for the blood glucose assay after fasting for 12 h, and plasma samples at the 4th and 8th weeks were collected for clinical biochemistry analyses. In addition, plasma samples were also collected at the 8th week for NMR analyses. The urine and feces samples were collected at the end of the 4th, 6th and 8th weeks with metabolic cages. The collection container was disinfected with 1% sodium azide (50 μL) before urine collection. All the samples were stored at −80 °C for NMR determination. All the rats were sacrificed at the end of the 8th week to remove the liver and kidney tissues for pathological examination.Clinical biochemistry and histopathology
Plasma biochemical analyses were carried out on an AMS-18 automatic biochemistry analyzer including alanine aminotransferase (ALT), aspartate aminotransferase (AST) and creatinine (CREA). An independent sample t test was conducted to compare the clinical biochemical data from the HT model and control rats. Liver and kidney tissues were fixed in 4% phosphate-buffered formaldehyde, and then they were embedded in paraffin wax and cut into 3 μm sections. Tissue sections were stained with hematoxylin and eosin (HE) and examined by light microscopy.Urine, plasma, and fecal sample preparation
The urine and plasma samples were thawed at room temperature just prior to NMR analysis. An aliquot (400 μL) of sample was mixed with phosphate buffer (150 μL, 0.2 M Na2HPO4/NaH2PO4, pH 7.4) to minimize chemical shift variations and then centrifuged (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g, 10 min at 4 °C) to remove any precipitates. The supernatant was then pipetted into a 5 mm NMR tube and D2O (80 μL) containing 0.05% sodium 3-trimethylsilyl-(2, 2, 3, 3-2H4)-1-propionate (TSP) was added.
000g, 10 min at 4 °C) to remove any precipitates. The supernatant was then pipetted into a 5 mm NMR tube and D2O (80 μL) containing 0.05% sodium 3-trimethylsilyl-(2, 2, 3, 3-2H4)-1-propionate (TSP) was added.
The fecal extract method for NMR analyses reported by Y. Zhao was used.26 Briefly, fecal extracts were prepared by mixing fecal samples (50 mg) with phosphate buffer (500 μL, 0.1 M K2HPO4/NaH2PO4 = 4/1, pH = 7.4) containing 30% D2O, 0.002% (w/v) TSP. After vortex mixing, the samples were subjected to three freeze-thaw treatments, followed by 10 ultrasonication cycles, and then the supernatant was collected. The residue was subjected to the same extraction method again. The residue supernatant was added to the initial supernatant and centrifuged at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 10 min at 4 °C. A portion of supernatant (550 μL) was transferred into a 5 mm NMR tube.
000g for 10 min at 4 °C. A portion of supernatant (550 μL) was transferred into a 5 mm NMR tube.
NMR spectroscopy
1H NMR spectra of all samples were collected at 298 K on a Bruker Avance III 500 MHz spectrometer. The NMR spectra of urine and fecal extract samples were recorded using the water-presaturated standard one-dimensional NOESYPR1D pulse sequence (recycle delay-90°-t1-90°-tm-90°-acquisition) to obtain representative total metabolite compositions. Sixty-four transients were collected into 32k data points using a spectral width of 10 kHz with a relaxation delay of 3 s and mixing time (tm) of 100 ms. t1 was set to 3 μs. The 1H NMR spectra of the plasma samples were recorded using the water-suppressed standard one-dimensional Carr–Purcell–Meiboom–Gill (CPMG) spin-echo pulse sequence (RD-90°-(τ-180°-τ)n-acquisition) in order to reduce the peak overlap. Sixty-four transients were collected into 32k data points using a spectral width of 10 kHz with a relaxation delay of 3 s, and the total echo time (2nτ) was 100 ms. The free-induction decays were multiplied by an exponential function with a line-broadening factor of 0.3 Hz before Fourier transformation. The chemical shifts of the spectra were referenced to the TSP signal at δ 0.00. To assist metabolite assignment, two-dimensional NMR (2D NMR) spectra such as 1H–1H correlation spectroscopy (COSY), total correlation spectroscopy (TOCSY) and 1H–13C heteronuclear single quantum coherence spectroscopy (HSQC) were carried out on the selected samples. COSY and TOCSY were performed with a total of 128 increments and 80 transients accumulated into 2048 data points with a spectral width of 10.5 ppm for both dimensions. The TOCSY 2D NMR spectra used a MLEV17 spin-lock duration of 90 ms. For HSQC, 2048 data points with 240 scans per increment and 120 increments were acquired with spectral widths of 10.0 and 150 ppm for 1H and 13C respectively.Pattern recognition and statistical analysis
All the spectra were phase- and baseline-corrected manually, and then bucketed and automatically integrated with an automation routine in AMIX. Each 1H NMR spectrum was segmented into regions of 0.005 ppm (urine and plasma) or 0.02 ppm (fecal extracts). The region δ 4.7–5.2 was discarded to eliminate the effects of water suppression. For the urine spectra, the region containing urea (δ 5.2–6.2) was also discarded to eliminate the urea signals. The integrals of these buckets covered the region δ 0.5–8.5 and were normalized to the total sum of the spectral integrals. The resulting normalized integral data obtained from urine, plasma, and feces samples were saved in Excel form, and then they were submitted to PCA and OPLS-DA using the software Simca-P+ 12.0 (Umetrics, Sweden). The scores plot, which highlights inherent clustering trends in the samples, and the loadings plot, which provides potential biomarkers, were both visualized. Statistical analyses were performed with an analysis of variance and p < 0.05 was identified as significant.Results
Influence of salt diet on weight, blood pressure, clinical biochemistry and histopathology
The salt diet had no effect on body weight and blood glucose during the whole period (Table 1). Significant changes in blood pressure were observed from the 6th week to the end of the 8th week, although there was no difference at the end of the 4th week between the control and model rats. This demonstrated that the high salt diet started to induce HT at the 6th week and it became severe at the 8th week, in accordance with the previous report.25 Plasma biochemistry results are presented in Table 1. Significant increases in plasma levels of AST and CREA were found at the end of the 8th week between the control and model rats, whereas there were no changes in the level of ALT. The histopathological examinations of liver and kidney tissues did not showed liver and kidney morphology changes (Fig. 1). The results demonstrated that 8 weeks of a high salt diet induced mild liver and kidney dysfunction, but did not affect the tissue morphology.| Index | 4th week | 6th week | 8th week | |||
|---|---|---|---|---|---|---|
| Control | Model | Control | Model | Control | Model | |
| a Values are presented as mean ± SD. *: indicates significant changes compared with controls *p < 0.05; **p < 0.01. /: indicates no measurement. | ||||||
| Weight (g) | 353.54 ± 24.53 | 348.85 ± 17.54 | 402.08 ± 19.12 | 400.68 ± 26.49 | 446.79 ± 22.36 | 439.24 ± 14.43 |
| Glucose (mmol L−1) | 5.86 ± 0.49 | 5.58 ± 0.78 | 5.53 ± 0.33 | 5.66 ± 0.45 | 5.77 ± 0.35 | 5.42 ± 1.55 |
| Pressure (mm Hg) | 110.72 ± 6.58 | 116.38 ± 4.76 | 112.17 ± 7.83 | 128.46 ± 4.95* | 111.03 ± 9.17 | 144.95 ± 5.87** |
| ALT (IU L−1) | 38.73 ± 5.24 | 42.04 ± 5.17 | / | / | 40.26 ± 6.25 | 49.83 ± 6.58 |
| AST (IU L−1) | 108.46 ± 14.78 | 119.74 ± 15.63 | / | / | 116.29 ± 15.32 | 152.47 ± 16.57** |
| CREA (μM L−1) | 37.66 ± 6.63 | 43.53 ± 6.17 | / | / | 41.59 ± 5.37 | 52.03 ± 9.82** |
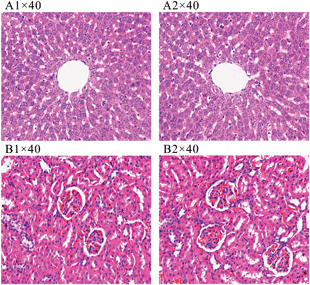 | ||
| Fig. 1 Histopathology of rat liver (A1: control, A2: model) and kidney (B1: control, B2: model) sections at the end of the 8th week. | ||
1H NMR spectroscopy and pattern recognition analysis of urine
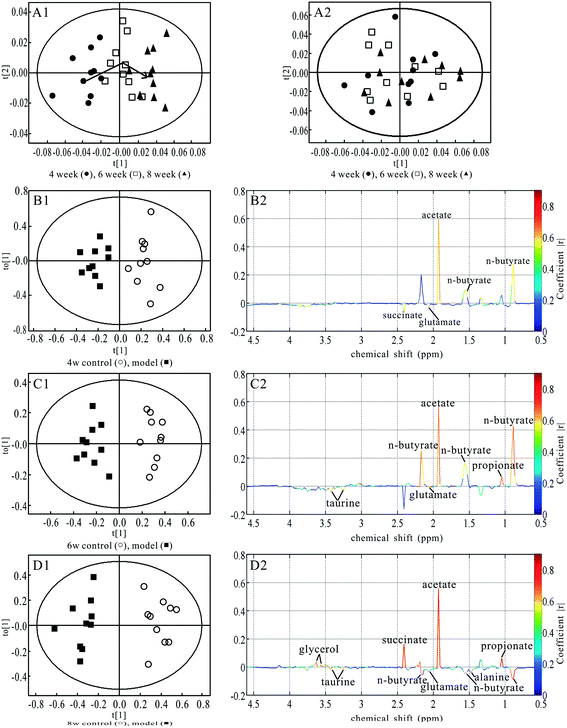
The representative 500 MHz 1H NMR NOESYPR1D spectra of urine from control and salt-fed HT groups at the 8th week are shown in Fig. 2. Assignments of endogenous metabolites were based on the literature27,28 and confirmed by 2D spectroscopy. The urinary NMR spectra were dominated by 2-hydroxybutyrate, 3-hydroxybutyrate, lactate, 2-hydroxyisobutyrate, alanine, acetate, N-acetylglycoprotein, acetone, acetoacetate, succinate, α-ketoglutaric acid (α-KG), citrate, dimethylamine (DMA), dimethylglycine (DMG), creatine, creatinine, N-acetylcarnitine, choline, phosphocholine (PC), glycerophosphocholine (GPC), trimethylamine-N-oxide (TMAO), betaine, taurine, 4-hydroxyphenylactate, glycine, glycerol, phosphoethanolamine, hippurate, N-methylnicotinamide, fumarate, phenylacetylglycine and formate. Visually, the urinary metabolic profiles did not showed distinct differences between the two groups. Therefore, we performed multivariate data analysis to determine the metabolic markers in HT model rats.Fig. 3A1 shows the scores plot of PCA representing the distribution of all the urinary samples at the end of the 4th, 6th and 8th weeks in HT group. The samples at the 4th week were separated from the ones at the 6th and 8th weeks along the t1 dimension. A classification between the 6th and 8th weeks was also observed as the arrow shows. The analysis showed that urine metabolic profiles might reflect metabonomic perturbations at different feeding times. PCA was also performed on the spectral data for the controls. The scores plot could not be classified for the samples at three time points (Fig. 3A2). The PCA results demonstrated that metabolic variations in model rats were closely correlated with high salt feeding.
OPLS-DA models were established for the classification of the controls and salt-fed HT rats in order to identify the associated potential biomarkers. In the correlation coefficient color-coded loadings plot, the hot colored metabolites (e.g., red) make a more significant contribution than the cold colored (e.g., blue) ones for the intergroup discrimination. The clear classifications between the control and HT rats at the 4th, 6th and 8th weeks are shown in the scores plots (Fig. 3B1, C1 and D1). The coefficient-coded loadings plot (Fig. 3B2) showed that the levels of creatine, creatinine, TMAO, betaine and hippurate increased, whereas those of α-KG, citrate and taurine decreased in the samples of the salt-fed model rats at the end of the 4th week. In addition to these metabolic changes, decreased levels of succinate, choline, PC and GPC were found at the end of the 6th and 8th weeks (Fig. 3C2 and D2). Table 2 summarizes the statistical analysis results of the normalized integrals of urine metabolites screened out in Fig. 3, accounting for the classification of the two groups at the three time points.
| Metabolites | Chemical shift | Variations | ||
|---|---|---|---|---|
| 4th week | 6th week | 8th week | ||
| a *: indicates significant changes compared with controls *p < 0.05; **p < 0.01. | ||||
| Succinate | 2.41(s) | — | ↓* | ↓* |
| α-KG | 2.45(t), 3.01(t) | ↓* | ↓* | ↓* |
| Citrate | 2.56(d), 2.72(d) | ↓* | ↓** | ↓** |
| Creatine + creatinine | 3.03(s) | ↑* | ↑** | ↑** |
| Creatinine | 4.05(s) | ↑* | ↑** | ↑** |
| Choline | 3.21(s) | — | ↓* | ↓** |
| PC + GPC | 3.23(s) | — | ↓* | ↓** |
| Taurine | 3.27(t), 3.43(t) | ↓* | ↓** | ↓** |
| TMAO + betaine | 3.27(s) | ↑* | ↑* | ↑** |
| Hippurate | 3.97(s), 7.54(t), 7.64(t), 7.84(d) | ↑* | ↑* | ↑** |
1H NMR spectroscopy and pattern recognition analysis of plasma
Typical 1H CPMG NMR spectra of plasma from control and model rats at the end of the 8th week were displayed in Fig. 2. The NMR signals were assigned according to the literature28,29 and confirmed by 2D spectroscopy. We observed the metabolites, including amino acids (leucine, isoleucine, valine, alanine and lysine), TCA cycle intermediate metabolites, lipids, lactate and glucose. The scores plot of OPLS-DA (Fig. 4A1) shows the clear separation between the controls and HT model rats. From the coefficient-coded loadings plot (Fig. 4A2), the elevated levels of LDL, VLDL, lactate, creatine, TMAO and betaine, and the decline in the levels of HDL, PC, GPC and glycerol, were observed in the plasma samples of the HT models at the end of the 8th week. Table 3 summarizes the statistical analysis results of the plasma metabolites screened out in Fig. 4, accounting for the differentiation between the two groups at the 8th week. | ||
| Fig. 4 OPLS-DA of plasma 1H NMR spectra data from control and salt-fed HT rats at the end of the 8th week (R2X = 77.3%, Q2Y = 73.2%). A1: scores plot; A2: coefficient-coded loadings plot. | ||
| Metabolites | Chemical shift | Variations |
|---|---|---|
| 8th week | ||
| a *: indicates significant changes compared with controls *p < 0.05;**p < 0.01. | ||
| HDL | 0.84(m) | ↓* |
| LDL + VLDL | 0.89(m), 1.29(m) | ↑* |
| Lactate | 1.33(d), 4.11(q) | ↑** |
| Creatine | 3.03(s) | ↑** |
| PC + GPC | 3.23(s) | ↓* |
| TMAO + betaine | 3.27(s) | ↑** |
| Glycerol | 3.56(dd) | ↓** |
1H NMR spectroscopy and pattern recognition analysis of fecal extracts
Typical 1H NMR NOESYPR1D spectra of fecal extracts from control and model rats at the 8th week are shown in Fig. 2. Endogenous metabolites were assigned based on the literature26 and confirmed by 2D spectroscopy. The main metabolites in the fecal extracts spectra were short chain fatty acids (SCFAs), such as butyrate, propionate and acetate, a series of amino acids, hemicellulosic sugars (arabinose and xylose), succinate and glucose. To detect further potential biomarkers in HT model rats, we performed multivariate data analysis.The PCA scores plot represents the distribution of model rats (Fig. 5A1), and a progressional classification was observed at the three time points indicated by the arrows, whereas the control group displayed no obvious differences (Fig. 5A2). The PCA results revealed that metabolic variations in model rats might reflect the effect of salt feeding.
OPLS-DA models were established for the classification of controls and salt-fed rats to detect further associated potential metabolic markers. The distinct classification of the control and HT rats at the 4th, 6th and 8th weeks is shown in the scores plots (Fig. 5B1, C1 and D1). The coefficient-coded loadings plot (Fig. 5B2) showed that the levels of glutamate and succinate increased, whereas n-butyrate and acetate decreased in the salt-fed models at the end of the 4th week. It was noted that increased taurine levels and decreased propionate levels were found at the end of the 6th week (Fig. 5C2), whereas succinate did not show significant changes. Alanine and n-butyrate levels increased in model rats at the end of the 8th week, whereas succinate and glycerol levels decreased (Fig. 5D2). Table 4 summarizes the statistical analysis results of the normalized integrals of the fecal extracts.
| Metabolites | Chemical shift | Variations | ||
|---|---|---|---|---|
| 4th week | 6th week | 8th week | ||
| a *: indicates significant changes compared with controls *p < 0.05; **p < 0.01. | ||||
| n-Butyrate | 0.90(t), 1.56(m), 2.16(t) | ↓* | ↓** | ↑* |
| Propionate | 1.06(t) | — | ↓** | ↓** |
| Alanine | 1.48(d) | — | — | ↑* |
| Acetate | 1.92(s) | ↓* | ↓** | ↓** |
| Glutamate | 2.06(m) | ↑* | ↑** | ↑** |
| Succinate | 2.41(s) | ↑* | — | ↓** |
| Taurine | 3.27(t), 3.43 (t) | — | ↑* | ↑** |
| Glycerol | 3.65(dd) | — | — | ↓** |
Discussion
Table 1 shows that excessive consumption of salt induced hypertension, which is in accordance with previous research.25,30 The plasma biochemistry data and histopathology evaluation showed mild liver and kidney dysfunction prior to obvious tissue histopathology damage caused by 8 weeks of a high salt diet. The salt-fed HT models used in our study have previously been found to be appropriate for studying hypertension associated with high salt consumption.31 We showed a series of metabolite changes in urine, plasma, and fecal extracts from salt-fed HT model rats by using a combination of pattern recognition techniques and1H NMR based metabonomics, revealing the systematic metabolic progression of HT and identifying the potential biomarkers for HT.A high salt diet disturbs energy metabolism
Energy generation in the body requires ATP, which mainly comes from glucose, glycolysis and lipid oxidation. Under normal physiological conditions, many substrates can be used, such as glucose, amino acids, fatty acids and ketone bodies. TCA cycle is the final metabolic pathway, which oxidizes and decomposes carbohydrates, amino acids and fatty acids, and it is also a critical link in the body's energy supply. The TCA cycle occurs in the mitochondria. Levels of succinate, citrate and α-KG, which are important intermediates in the TCA cycle, continuously decreased in salt-fed rats, possibly as a result of the inhibition of the Kreb's cycle. This suggested that energy metabolism was disturbed32 and that mitochondrial function was impaired33 by the high salt diet. YIT 12067T-like bacteria are distributed widely in the gastrointestinal tract because they are specialized to use succinate.34 The levels of succinate increased in the 4th week and decreased in the 8th week, which probably indicated the disordered activity of intestinal bacteria that use succinate. The plasma lactate in the model group increased sharply, which suggested that metabolism is directed away from the TCA cycle, which would promote lactate production through glycolysis.A high salt diet induces liver and kidney dysfunction
Taurine (2-aminoethanesulfonic acid) is the major intracellular free β-amino acid and plays several physiological roles including antioxidation, osmoregulation, membrane stabilization, and attenuation of apoptosis.35,36 The kidneys are the main organ that excretes taurine, which is regulated based on the needs of the body and dietary taurine content. The liver is the main organ that synthesizes taurine, and urinary taurine is associated with liver toxicity and damage.37 The decrease in urinary taurine during the whole experimental period may arise from liver and kidney dysfunction induced by excessive consumption of salt, which is consistent with the clinical chemistry results. The level of fecal taurine increased between the 6th and 8th weeks. This could be explained by a decrease in the activity of intestinal bacteria that use taurine, which resulted in the taurine accumulation in feces, although this conjecture requires further verification. Studies have reported that a high salt diet can also induce hepatic steatosis.38 However, the liver histopathology showed no obvious morphological changes in this study. Therefore, 8 weeks of high salt diet caused mild liver and kidney dysfunction prior to damage obvious in the tissue histopathology.Muscle motion requires a large amount of energy, and creatinine is an important compound that is produced by the decomposition of creatine and is excreted by kidney. Tang et al.39 reported that the decreased level of urinary creatine and creatinine indicated impaired glomerular filtration, which was verified by the histopathologically observed vascular dilatation and congestion in the nephrons. In our study, increased urinary creatine and creatinine levels were found in model rats compared with the controls. These changes indicated that administration of a high salt diet possibly induced vasoconstriction and even glomerular inflammation.
A high salt diet disturbs lipid metabolism
HDL is the smallest lipoprotein that transports cholesterol from extra hepatic tissues to the liver for excretion, and is thought to be anti-atherogenic. Elevated HDL levels confer a decreased risk of coronary heart disease (CHD)40 on an individual. In this regard, HDL is often referred to as “good cholesterol”, and higher levels are considered beneficial. In contrast, LDL and VLDL are considered to be “bad cholesterol”, and transport about 75% of the body's cholesterol to the cells. The concentration of LDL and VLDL is associated with atherosclerosis.41 Bimenya and co-workers reported that a high LDL/HDL ratio is a good predictor for cardiovascular disease.42 It was also reported that a high salt intake induced a significant decrease in HDL, which proved that excessive salt consumption is associated with lipid metabolism disturbances.10 Therefore, low levels of HDL in conjunction with higher levels of LDL and VLDL in plasma at the end of the 8th week could suggest an increased risk of cardiovascular diseases from a high salt diet. This is consistent with the research published by Yu et al.43 that showed that an 8% high salt diet results in vascular dysfunction and hypertension with compensatory cardiac hypertrophy.A high salt diet disturbs choline metabolism
Choline is an important precursor of phosphocholine, which is activated by choline kinase and phosphocholine cytidylyltransferase. The substantial decreases in choline, PC and GPC, which are major components of cell membranes, were found in urine at the end of the 6th week and decreased significantly at the 8th week, and also decreased in plasma at the 8th week. This demonstrated the membrane phosphocholine metabolism was disturbed during the period of HT. Choline is an important source for methyl groups via one of its metabolites, betaine, that participates in the S-adenosylmethionine synthesis pathways.44 So the decline in the level of choline probably resulted in the accumulation of betaine in the urine and plasma.A high salt diet disturbs gut microbiota
Acetate, propionate, and butyrate are the primary short chain fatty acids (SCFAs) produced by gut bacteria from carbohydrates. Dolara et al.45 have demonstrated that lower concentrations of SCFAs in feces are associated with higher rates of colonic mucosal proliferation, which is directly related to an increased risk of colon cancer. The clear decrease in acetate and propionate in fecal extracts revealed that the high salt diet probably altered the gut bacteria and colonic mucosal proliferation. N-Butyrate is generally produced from colonic carbohydrate fermentation by microbiota, and it is regarded as an important energy source for the colonic epithelial cells of the host.46 In fecal extracts, the levels of n-butyrate decreased at the 4th and 6th weeks, whereas it increased at the end of the 8th week, which also indicated that high salt feeding induced colonic microbiota disturbance.Many strains of gut bacteria decompose choline to trimethylamine (TMA), which is then oxidized to TMAO prior to excretion.47 The precursor of hippurate is produced by gut bacteria, and it is correlated with the microbial activity and composition of the gut.48,49 A time-dependent increase in hippurate levels in urine and TMAO indicates that the gut microbiota are disturbed during salt feeding. The increased level of TMAO in plasma also indicates the disorder of intestinal flora. The higher levels of glutamate in the feces of high salt-fed rats are probably caused by increases in dietary protein degradation or amino acid biosynthesis because of increased bacterial populations in the feces.50 Glutamate is a vital nutrient in humans and animals.51 Many factors can influence the levels of glutamate in fecal extracts, such as the absorption of the gut epithelium and the metabolism of gut microbiota. The increased levels of glutamate in fecal extracts from the 4th week to the end of the 8th week potentially suggest gut epithelium dysfunction and disordered intestinal flora. It has been reported that high alanine levels in feces are associated with the presence of uracil, which can be decomposed to alanine by some intestinal bacteria.51 The elevated alanine in feces samples of salt-fed rats at the end of the 8th week could show that the bacterial degradation of uracil to alanine increased.
Conclusions
This study has contributed to the metabolic transfer understanding of hypertension induced by a high salt diet. The time-dependent changes in endogenous metabolites caused by this diet pattern were identified. NMR metabonomics together with multivariate analyses of urine, plasma, and feces revealed a number of complex disturbances in the endogenous metabolites, which revealed progression axes from normal status to HT status. Lipid metabolism, energy, and gut microbiota metabolism, along with liver and kidney dysfunction, could be affected by salt feeding. We conclude that 1H NMR-based metabonomics is a useful method for discovering relevant biochemical changes to monitor the progression of hypertension caused by high salt feeding from a systematic and holistic view.Acknowledgements
We acknowledge the financial supports from the National Natural Science Foundation of China (21005022), NSFC-Guangdong joint fund (no. U1132001), and the Guangdong Provincial Department of Science and Technology fund (no. 2011B031700018). We acknowledge Mrs Xiaoling Guo for her valuable technical assistance.References
- Y. Yano and K. Kario, Hypertens. Res., 2012, 35, 695–701 CrossRef PubMed.
- P. M. Kearney, M. Whelton, K. Reynolds, P. Muntner, P. K. Whelton and J. He, Lancet, 2005, 365, 217–223 CrossRef.
- E. D. Frohlich and J. Varagic, Curr. Opin. Cardiol., 2005, 20, 424–429 CrossRef.
- P. Meneton, X. Jeunemaitre, H. E. de Wardener and G. A. MacGregor, Physiol. Rev., 2005, 85, 679–715 CrossRef CAS PubMed.
- M. H. Weinberger, Hypertension, 1996, 27, 481–490 CrossRef CAS.
- N. R. Poulter, K. T. Khaw, B. E. Hopwood, M. Mugambi, W. S. Peart, G. Rose and P. S. Sever, BMJ, 1990, 300, 967–972 CrossRef CAS.
- R. Baudrand, C. Campino, C. A. Carvajal, O. Olivieri, G. Guidi, G. Faccini, P. A. Vöhringer, J. Cerda, G. Owen, A. M. Kalergis and C. E. Fardella, Clin. Endocrinol., 2014, 80, 677–684 CrossRef CAS PubMed.
- E. Tchistiakova, N. D. Anderson, C. E. Greenwood and B. J. Maclntosh, Neuroimage Clin., 2014, 5, 36–41 CrossRef PubMed.
- A. D. Colosia, R. Palencia and S. Khan, Diabetes, Metab. Syndr. Obes.: Targets Ther., 2013, 6, 327–338 CrossRef PubMed.
- S. A. González, P. Forcada, E. M. de Cavanagh, F. Inserra, J. C. Svane, S. Obregón, C. Castellaro, D. Olano, A. Hita and C. V. Kotliar, Am. J. Hypertens., 2012, 25, 620–624 CrossRef PubMed.
- X. Kong, D. Y. Zhang, H. B. Wu and F. X. Li, Biol. Pharm. Bull., 2011, 34, 693–699 CAS.
- H. Cochet, W. Lefrancois, M. Montaudon, F. Laurent, L. Pourtau, S. Miraux, E. Parzy, J. M. Franconi and E. Thiaudière, Eur. Radiol., 2013, 23, 332–338 CrossRef PubMed.
- L. S. Bilbis, S. A. Muhammad, Y. Saidu and Y. Adamu, Biochem. Res. Int., 2012, 2012, 678582 CAS.
- C. J. Rodriguez, K. Bibbins-Domingo, Z. Jin, M. L. Daviglus, D. C. Goff Jr and D. R. Jacobs Jr, Hypertension, 2011, 58, 410–416 CrossRef CAS PubMed.
- P. Le Corvoisier, C. Adamy, L. Sambin, B. Crozatier, A. Berdeaux, J. B. Michel, L. Hittinger and J. Su, Eur. J. Heart Failure, 2010, 12, 1171–1178 CrossRef CAS PubMed.
- J. K. Nicholson, J. C. Lindon and E. Holmes, Xenobiotica, 1999, 29, 1181–1189 CrossRef CAS PubMed.
- P. Wang, H. Sun, H. T. Lv, W. J. Sun, Y. Yuan, Y. Han, D. W. Wang, A. H. Zhang and X. J. Wang, J. Pharm. Biomed. Anal., 2010, 53, 631–645 CrossRef CAS PubMed.
- J. K. Nicholson and I. D. Wilson, Prog. Nucl. Magn. Reson. Spectrosc., 1989, 21, 449–501 CrossRef CAS.
- S. Schrading, H. Schild, M. Kühr and C. Kuhl, Radiology, 2014, 271, 45–55 CrossRef PubMed.
- L. C. Zhao, X. Liu, L. Y. Xie, H. C. Gao and D. H. Li, Anal. Sci., 2010, 26, 1277–1282 CrossRef CAS.
- J. Y. Jung, I. Y. Kim, Y. N. Kim, J. S. Kim, J. H. Shin, Z. H. Jang, H. S. Lee, G. S. Hwang and J. K. Seong, BMB Rep., 2012, 45, 419–424 CrossRef CAS.
- J. B. Peng, H. M. Jia, T. Xu, Y. T. Liu, H. W. Zhang, L. L. Yu, D. Y. Cai and Z. M. Zou, Process Biochem., 2011, 46, 2240–2247 CrossRef CAS PubMed.
- P. Bernini, I. Bertini, C. Luchinat, L. Tenori and A. Tognaccini, J. Proteome Res., 2011, 10, 4983–4992 CrossRef CAS PubMed.
- S. Seijo, J. J. Lozano, C. Alonso, E. Reverter, R. Miquel, J. G. Abraldes, M. L. Martinez-Chantar, A. Garcia-Criado, A. Berzigotti, A. Castro, J. M. Mato, J. Bosch and J. C. Garcia-Pagan, Am. J. Gastroenterol., 2013, 108, 926–932 CrossRef CAS PubMed.
- J. W. Gu, E. Young, Z. J. Pan, K. B. Tucker, M. Shparago, M. Huang and A. P. Bailey, Beijing Daxue Xuebao, Yixueban, 2009, 41, 505–515 CAS.
- Y. Zhao, J. F. Wu, J. V. Li, N. Y. Zhou, H. R. Tang and Y. L. Wang, J. Proteome Res., 2013, 12, 2987–2999 CrossRef CAS PubMed.
- X. J. Zhao, C. Y. Huang, H. H. Lei, X. Nie, H. R. Tang and Y. L. Wang, J. Proteome Res., 2011, 10, 5183–5190 CrossRef CAS PubMed.
- C. Y. Jiang, K. M. Yang, L. Yang, Z. X. Miao, Y. H. Wang and H. B. Zhu, PLoS One, 2013, 8, e66786 CAS.
- J. K. Nicholson, P. J. D. Foxall, M. Spraul, R. D. Farrant and J. C. Lindon, Anal. Chem., 1995, 67, 793–811 CrossRef CAS.
- Y. Hara, A. Noda, S. Miyata, M. Minoshima, M. Sugiura, J. Kojima, M. Otake, M. Furukawa, X. W. Cheng, K. Nagata and T. Murohara, Exp. Anim., 2013, 62, 305–310 CrossRef CAS.
- Y. Naito, H. Sawada, M. Oboshi, A. Fujii, S. Hirotani, T. Iwasaku, Y. Okuhara, A. Eguchi, D. Morisawa, M. Ohyanagi, T. Tsujino and T. Masuyama, PLoS One, 2013, 8, e75906 CAS.
- W. J. Cong, Q. L. Liang, L. Li, J. Shi, Q. F. Liu, Y. Feng, Y. M. Wang and G. A. Luo, Talanta, 2012, 89, 91–98 CrossRef CAS PubMed.
- N. J. Waters, C. J. Waterfield, R. D. Farrant, E. Holmes and J. K. Nicholson, Chem. Res. Toxicol., 2005, 18, 639–654 CrossRef CAS PubMed.
- Y. Watanabe, F. Nagai and M. Morotomi, Appl. Environ. Microbiol., 2012, 78, 511–518 CrossRef PubMed.
- C. Condron, P. Neary, D. Toomey, H. P. Redmond and D. Bouchier-Hayes, Shock, 2003, 19, 564–569 CrossRef CAS PubMed.
- S. G. Maher, C. E. Condron, D. J. Bouchier-Hayes and D. M. Toomey, Clin. Exp. Immunol., 2005, 139, 279–286 CrossRef CAS PubMed.
- C. J. Waterfield, J. A. Turton, M. D. Scales and J. A. Timbrell, Arch. Toxicol., 1993, 67, 244–254 CrossRef CAS.
- S. Arima, H. Uto, R. Ibusuki, R. Kumamoto, S. Tanoue, S. Mawatari, K. Oda, M. Numata, H. Fujita, M. Oketani, A. Ido and H. Tsubouchi, Int. J. Mol. Med., 2014, 33, 68–76 CAS.
- B. W. Tang, J. J. Ding, F. H. Wu, L. Chen, Y. X. Yang and F. Y. Song, J. Ethnopharmacol., 2012, 141, 134–142 CrossRef CAS PubMed.
- J. J. Genest, J. R. McNamara, D. N. Salem and E. J. Schaefer, Am. J. Cardiol., 1991, 67, 1185–1189 CrossRef CAS.
- W. P. Castelli, R. J. Garrison, P. W. Wilson, R. D. Abbott, S. Kalousdian and W. B. Kannel, JAMA, J. Am. Med. Assoc., 1986, 256, 2835–2838 CrossRef CAS.
- G. S. Bimenya, J. K. Okot, H. Nangosa, S. A. Anguma and W. Byarugaba, Afr. J. Health Sci., 2006, 6, 139–144 CAS.
- Q. L. Yu, D. F. Larson, D. Slayback, T. F. Lundeen, J. H. Baxter and R. R. Watson, Cardiovasc. Toxicol., 2004, 4, 37–46 CrossRef PubMed.
- P. I. Holm, P. M. Ueland, G. Kvalheim and E. A. Lien, Clin. Chem., 2003, 49, 286–294 CAS.
- P. Dolara, G. Caderni, M. Salvadori, G. Morozzi, R. Fabiani, A. Cresci, C. Orpianesi, G. Trallori, A. Russo and D. Palli, Nutr. Cancer, 2002, 42, 186–190 CrossRef CAS PubMed.
- T. L. Miller and M. J. Wolin, Appl. Environ. Microbiol., 1996, 62, 1589–1592 CAS.
- J. R. Baker and S. J. Chaykin, Biol. Chem., 1962, 237, 1309–1313 CAS.
- N. G. Psihogios, I. F. Gazi, M. S. Elisaf, K. I. Seferiadis and E. T. Bairaktari, NMR Biomed., 2008, 21, 195–207 CrossRef CAS PubMed.
- L. Wei, P. Q. Liao, H. F. Wu, X. J. Li, F. K. Pei, W. S. Li and Y. J. Wu, Toxicol. Appl. Pharmacol., 2009, 234, 314–325 CrossRef CAS PubMed.
- Y. S. Hong, Y. T. Ahn, J. C. Park, J. H. Lee, H. Lee, C. S. Huh, D. H. Kim, H. Ryu do and G. S. Hwang, Arch. Pharmacal Res., 2010, 33, 1091–1101 CrossRef CAS PubMed.
- G. Andersen, B. Andersen, D. Dobritzsch, K. D. Schnackerz and J. Piskur, FEBS J., 2007, 274, 1804–1817 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |