Nucleophilic radiofluorination at room temperature via aziridinium intermediates†
Abstract
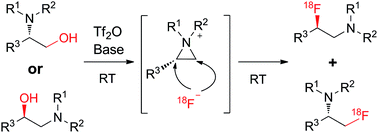
β-[18F]fluoroamines were radiolabeled using anchimeric assistance of the amine. The ring opening of the aziridinium intermediate by different sources of nucleophilic fluoride at RT led to both fluorinated regioisomers with 18F-incorporation yields of up to 77% at RT. The radiofluorination 2-[18F]fluoroethylamines afforded single compounds from the alcohol precursor at RT.

 Please wait while we load your content...
Please wait while we load your content...