Stability and interaction forces of oil-in-water emulsions as observed by optical tweezers – a proof-of-concept study
Abstract
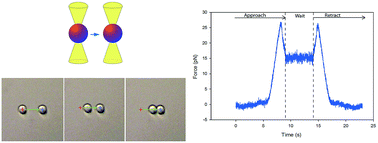
Increased insight into the interactions occurring between emulsion droplets is important to a range of applications from the food and pharmaceutical industries to oil recovery and mineral flotation. These interactions are often modified by the adsorption at the oil–water interface of surface-active species such as small molecule surfactants, proteins or polymers, in order to meet functional requirements of the emulsions. However, the experimental challenges faced when attempting to study these forces acting between emulsion droplets have hampered the progress in the understanding of the fundamental forces and to which extent these forces influence the destabilizing processes. In this paper we describe emulsion droplet studies by applying optical tweezers. By capturing two emulsion droplets in separate optical traps and bringing them into proximity, the forces acting between them can be measured as a function of separation distance. In this proof-of-concept study the force versus distance curves of emulsion droplets of different stabilization was obtained. Focus has been placed on the relative differences between micro- and macromolecular stabilization of emulsion droplets. Effects on depletion interaction, relaxation behaviour of the interfacial polymer layer during compression of the droplets and electrostatic screening have been observed. The present article documents the suitability of optical tweezers in studies aiming at revealing the forces acting between individual emulsion droplets as well as limiting factors of the technology.

 Please wait while we load your content...
Please wait while we load your content...