Facile synthesis of Co3O4 porous nanosheets/reduced graphene oxide composites and their excellent supercapacitor performance†
Abstract
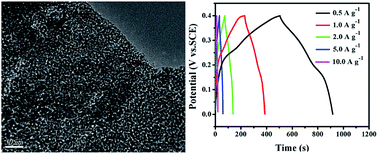
Co3O4/RGO composites with Co3O4 porous nanosheets attached on reduced graphene oxide (RGO) sheet were fabricated through a facile refluxing method followed by a thermal annealing process. Pores with sizes of several nanometers are uniformly distributed in Co3O4 nanosheets. The composites as electrode materials for supercapacitors were investigated. They deliver a specific capacitance as high as 518.8 F g−1 at the current density of 0.5 A g−1, and great cycling stability with a decay of about 7.6% after 1500 continuous charge–discharge cycles at the current density of 10 A g−1. The excellent capacitive performance can be attributed to the excellent electrical properties, large surface area and well-connected conductive network derived from the structural advantages of the Co3O4 porous nanosheets and RGO support. The facile synthesis and the excellent capacitive performance make the Co3O4/RGO composites a promising candidate for electrode materials in electrochemical energy storage systems.

 Please wait while we load your content...
Please wait while we load your content...