Synthesis and characterization of amphiphilic triblock Copolymers with Identical compositions but different block sequences†
Chengjiao Lu,
Lingdi Chen,
Kun Huang and
Guowei Wang*
Department of Macromolecular Science, State Key Laboratory of Molecular Engineering of Polymers, Fudan University, Shanghai 200433, China. E-mail: gwwang@fudan.edu.cn
First published on 29th August 2014
Abstract
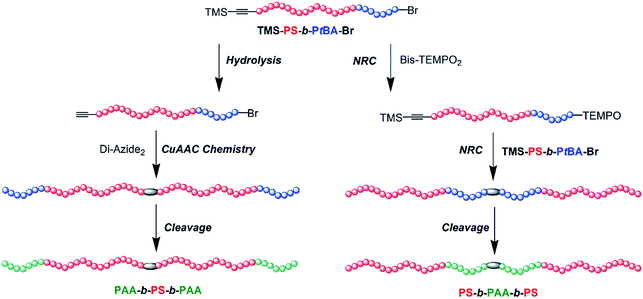
The amphiphilic triblock copolymers poly(acrylic acid)-b-poly(styrene)-b-poly(acrylic acid) (PAA-b-PS-b-PAA) and PS-b-PAA-b-PS with identical compositions but different block sequences were synthesized by a combination of an atom transfer radical polymerization (ATRP) mechanism and a nitroxide radical coupling (NRC) reaction or copper-catalyzed azide/alkyne click (CuAAC) chemistry. Firstly, the diblock copolymers TMS–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –PS-b-PtBA-Br were prepared by sequential ATRP of styrene (St) and tert-butyl acrylate (tBA) monomers from trimethylsilyl propargyl 2-bromoisobutyrate (TMS-PgBiB) initiator. Then, the triblock copolymers PS-b-PtBA-b-PS were prepared by NRC reaction between TMS–
–PS-b-PtBA-Br were prepared by sequential ATRP of styrene (St) and tert-butyl acrylate (tBA) monomers from trimethylsilyl propargyl 2-bromoisobutyrate (TMS-PgBiB) initiator. Then, the triblock copolymers PS-b-PtBA-b-PS were prepared by NRC reaction between TMS–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –PS-b-PtBA-Br and coupling agent bis[4-(2,2,6,6-tetramethylpiperidine-1-oxyl)] succinate (Bis-TEMPO2). And the triblock copolymers PtBA-b-PS-b-PtBA were obtained by CuAAC chemistry between Alkynyl-PS-b-PtBA-Br and a coupling agent 1,4-diazidobutane (Di-Azide2). The target triblock copolymers PAA-b-PS-b-PAA and PS-b-PAA-b-PS were finally derived from the cleavage of the corresponding PS-b-PtBA-b-PS and PtBA-b-PS-b-PtBA. The self-assembly behaviour was preliminarily studied by FLS, FESEM and DLS instruments, and the results showed that the PS-b-PAA-b-PS and PAA-b-PS-b-PAA could give distinct critical micelle concentration (cmc) values and different sizes of micelles in water.
–PS-b-PtBA-Br and coupling agent bis[4-(2,2,6,6-tetramethylpiperidine-1-oxyl)] succinate (Bis-TEMPO2). And the triblock copolymers PtBA-b-PS-b-PtBA were obtained by CuAAC chemistry between Alkynyl-PS-b-PtBA-Br and a coupling agent 1,4-diazidobutane (Di-Azide2). The target triblock copolymers PAA-b-PS-b-PAA and PS-b-PAA-b-PS were finally derived from the cleavage of the corresponding PS-b-PtBA-b-PS and PtBA-b-PS-b-PtBA. The self-assembly behaviour was preliminarily studied by FLS, FESEM and DLS instruments, and the results showed that the PS-b-PAA-b-PS and PAA-b-PS-b-PAA could give distinct critical micelle concentration (cmc) values and different sizes of micelles in water.
Introduction
The rapid development of “living”/controlled polymerization mechanisms and efficient coupling techniques in polymer chemistry have greatly accelerated the synthesis of polymers with complicated architectures,1–7 which further makes it possible to explore the structure–property relationship of polymers. For example, as an important research area, the research on self-assembly behaviour of amphiphilic polymers with complicated architectures has been largely beneficial from the progresses in polymer chemistry and especially focused by numerous scientists, and appreciable improvement has been achieved.Originally, Eisenberg et al. reported that the diblock copolymers, for example of poly(styrene)-b-poly(acrylic acid) (PS-b-PAA), could form a variety of interesting morphologies like spheres,8–10 rods,11,12 lamellas,13–15 vesicles8,16,17 and hexagonally packed hollow hoops18 when the medium was deliberately selected. As the simplest topology of diblock copolymers in various complicated polymers, the effect of topology on self-assembly behaviour of polymers might not be reflected in such system. The research model with somewhat complicated topology might be a better choice, for example of PAA-b-PS-b-PAA, different toroid-shaped and spherical morphologies could be observed in selective solvents.19 Followed with the pioneer work of Eisenberg's, considerable research progresses have also been made by other researchers. However, most researches were focused on the effect of solvents, compositions, topologies on self-assembly behaviour with multiple independent variables changing at the same time,14,20–23 and limited works were concentrated on the comparative study of polymers changing a single variable at once (for example, the same compositions but different topologies or conversely the same topologies but different compositions, etc.). In our previous work, we had tried to synthesize some triblock copolymer poly(styrene)-poly(ethylene oxide)-poly(acrylic acid) (PS-b-PEO-b-PAA) and PEO-b-PS-b-PAA with different block sequences to investigate and compare their self-assembly behaviours.20 Although the polymeric segments could be regulated in the different sequences by a certain synthetic procedure, limited in the synthetic methods, the molecular weight of each segment was difficult to control and the obtained results could not be compared in a parallel level. Thus, one could find that the synthesis of polymers with precise compositions and topologies is still a key point and a challenging work.
Typically, for the copolymer with certain compositions and topologies, the “living”/controlled polymerization mechanisms and efficient coupling techniques must be elaborately combined, rather than a random procedure. Especially, a certain efficient coupling technique always plays an important role in the synthesis of polymers. Copper-catalyzed azide/alkyne click (CuAAC) chemistry, because of its tolerance to solvents and various functional groups, as well as its high efficiency (close to 100%),24 has been widely applied to the synthesis of multi-block, star-like, hyperbranched, dendrimer-like polymers, etc.25–28 Another coupling reaction developed by our group, nitroxide radical coupling (NRC) reaction, has also been proved to be a rather robust and orthogonal technique with almost 100% efficiency.29–34 Because of the easily available functional halogen groups from atom transfer radical polymerization (ATRP) mechanism, and nitroxide radicals (such as the 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) group or its derivatives) from anionic polymerization mechanism or further modification, the NRC reaction has become an important candidate of coupling technique in polymer chemistry. By means of this NRC reaction, a library of polymers with complicated architectures has also been smoothly realized.30–32
Considering the above researches in self-assembly and the synthetic strategies to complicated polymers, in this contribution, by combination of ATRP mechanism and efficient NRC reaction or CuAAC chemistry, an innovative synthetic process to triblock copolymers PS-b-PAA-b-PS and PAA-b-PS-b-PAA with identical compositions but different block sequences was explored. The key precursor of diblock copolymer (TMS-PS-b-PtBA-Br) with a bromide group and a trimethylsilyl (TMS) protected alkynyl groups was firstly obtained by sequential ATRP mechanism. Then, the NRC reaction and CuAAC chemistry were selectively used to synthesize triblock copolymer PS-b-PtBA-b-PS and PtBA-b-PS-b-PtBA, respectively, and PS-b-PAA-b-PS and PAA-b-PS-b-PAA were further obtained by cleavage of PtBA segment (Scheme 1). In order to study the difference between PS-b-PAA-b-PS and PAA-b-PS-b-PAA, the critical micelle concentration (cmc) and the morphologies of copolymers in water were preliminarily investigated and compared.
Experimental
Materials
Styrene [St, 99%, Sinopharm Chemical Reagent Co. (SCR)] was washed with 10% NaOH aqueous solution followed by water three times, dried over anhydrous MgSO4, further dried over CaH2, and distilled under reduced pressure. tert-Butyl acrylate (tBA, 99%, SCR) was dried by CaH2 for 24 h and distilled under reduced pressure before use. Tetrahydrofuran (THF, >99%, SCR) was refluxed and distilled from sodium naphthalenide solution. Copper(I) bromide [Cu(I)Br, 95%, SCR] was stirred overnight in acetic acid, filtered, washed with ethanol and ethyl ether successively, and dried in vacuum. N-Phenyl-1-naphthylamine (PNA, 97%, Alfa Aesar) was purified by recrystallization in ethanol for three times. Trimethylsilyl propargyl alcohol (99%), 2-bromoisobutyryl bromide (99%), 1,4-dibromobutane (98%), N,N,N′,N′′,N′′-pentamethyldiethylenetriamine (PMDETA, 99%) and tetrabutylammonium fluoride hydrate (TBAF, 98%) were purchased from Aldrich and used as received. Tris((N,N,-dimethylamino)ethyl)amine (Me6TREN, 99%) was purchased from Alfa Aesar and used as received. Dimethyl sulfoxide (DMSO, 99%), dimethyl formamide (DMF, 99.5%) and sodium azide (NaN3, 98%) were purchased from SCR and used as received. Trimethylsilyl propargyl-2-bromoisobutyrate (TMS-PgBiB) was synthesized according to literature.35 4-Hydroxyl-2,2,6,6-tetramethylpiperidine-1-oxyl (HO-TEMPO) was synthesized according to literature.36 Bis[4-(2,2,6,6-tetramethylpiperidine-1-oxyl)] succinate (Bis-TEMPO2) was synthesized according to literature from HO-TEMPO and succinyl dichloride.37 1,4-diazidobutane (Di-Azide2) was synthesized according to literature38 from 1,4-dibromobutane and NaN3. All other reagents were all purchased from SCR and used as received except for declaration.Measurements
Gel permeation chromatographic (GPC) was performed in THF at 35 °C with an elution rate of 1.0 mL min−1 on an Agilent 1100 equipped with a G1310A pump, a G1362A refractive index detector, and a G1314A variable wavelength detector. One 5 μm LP gel column (500 Å, molecular weight range 500–2 × 104 g mol−1) and two 5 lm LP gel mixed bed columns (molecular weight range 200–3 × 106 g mol−1) were calibrated by PS standard samples. 1H NMR spectra were recorded on a DMX 500 MHz spectrometer in CDCl3 with tetramethylsilane (TMS) as the internal reference, except for the products PS-b-PAA-b-PS and PAA-b-PS-b-PAA, which were measured in DMF-d7 solvent. Steady-state fluorescent spectra of PNA were measured on a FLS920 spectrometer operating at 25 °C. The emission intensity at 418 nm was recorded to determine the cmc and the λex was 340 nm. The size of the micelles was measured by dynamic light scattering (DLS) using a Malvern Zetasizer Nano ZS. Field emission scanning electron microscopy (FESEM) images were obtained by an Ultra 55 field-emission scanning electron microscope operated at 5 kV.![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e002.gif) –PS-Br). The heterofunctional TMS–
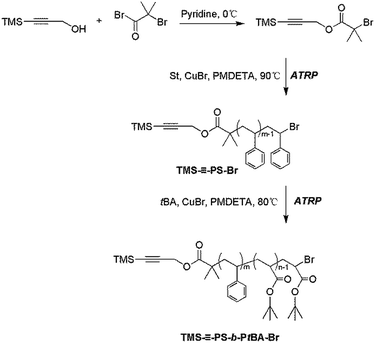
–PS-Br). The heterofunctional TMS–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –PS-Br was obtained by ATRP of St monomers from TMS-PgBiB initiator (Scheme 2). Typically, TMS-PgBiB (0.4610 g, 1.66 mmol), CuBr (0.2450 g, 1.71 mmol), PMDETA (0.35 mL, 1.68 mmol) and St (40 mL, 348 mmol) were introduced into a 100 mL ampule successively. The reaction mixture was degassed by three freeze–pump–thaw cycles and purged with nitrogen, and then immerged into an oil bath at 90 °C for 5.0 h. After the polymerization was stopped by dipping into liquid nitrogen, the crude product was diluted with THF and passed through a column chromatograph filled with neutral alumina to remove the copper complex, and finally precipitated into methanol. The precipitate was collected and dried at 45 °C in vacuum to a constant weight. GPC: Mn,GPC = 7500 g mol−1, PDI = 1.06. 1H NMR (CDCl3, δ, ppm, TMS): 0.12–0.22 ((CH3)3Si–), 0.78–0.98 (–C(CH3)2–), 1.17–2.51 (3H, aliphatic main chain –CH2CH– on PS chain), 3.95–4.20 (–C
–PS-Br was obtained by ATRP of St monomers from TMS-PgBiB initiator (Scheme 2). Typically, TMS-PgBiB (0.4610 g, 1.66 mmol), CuBr (0.2450 g, 1.71 mmol), PMDETA (0.35 mL, 1.68 mmol) and St (40 mL, 348 mmol) were introduced into a 100 mL ampule successively. The reaction mixture was degassed by three freeze–pump–thaw cycles and purged with nitrogen, and then immerged into an oil bath at 90 °C for 5.0 h. After the polymerization was stopped by dipping into liquid nitrogen, the crude product was diluted with THF and passed through a column chromatograph filled with neutral alumina to remove the copper complex, and finally precipitated into methanol. The precipitate was collected and dried at 45 °C in vacuum to a constant weight. GPC: Mn,GPC = 7500 g mol−1, PDI = 1.06. 1H NMR (CDCl3, δ, ppm, TMS): 0.12–0.22 ((CH3)3Si–), 0.78–0.98 (–C(CH3)2–), 1.17–2.51 (3H, aliphatic main chain –CH2CH– on PS chain), 3.95–4.20 (–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–CH2O–), 4.35–4.55 (BrCHC6H5–), 6.30–7.30 (5H, aromatic –C6H5 on PS chain). Mn,NMR = 7400 g mol−1.
C–CH2O–), 4.35–4.55 (BrCHC6H5–), 6.30–7.30 (5H, aromatic –C6H5 on PS chain). Mn,NMR = 7400 g mol−1.
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e002.gif) –PS-b-PtBA-Br). The diblock copolymer TMS–
–PS-b-PtBA-Br). The diblock copolymer TMS–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –PS-b-PtBA-Br was obtained by ATRP of tBA monomers initiated from TMS-PS-Br macro-initiator (Scheme 2). First, TMS–
–PS-b-PtBA-Br was obtained by ATRP of tBA monomers initiated from TMS-PS-Br macro-initiator (Scheme 2). First, TMS–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –PS-Br (3.00 g, 0.40 mmol), CuBr (0.0630 g, 0.44 mmol), PMDETA (0.10 mL, 0.48 mmol), tBA (20 mL, 138 mmol) and toluene (12 mL) were sequentially introduced into a 100 mL ampule. The reaction mixture was degassed by three freeze–pump–thaw cycles and purged with nitrogen, and immerged into an oil bath at 80 °C for 5.0 h. After the polymerization was stopped by dipping into liquid nitrogen, the crude product was diluted with THF and passed through a column chromatograph filled with neutral alumina to remove the copper complex, and precipitated into the mixed solvents of methanol and water (Vmethanol/Vwater = 1
–PS-Br (3.00 g, 0.40 mmol), CuBr (0.0630 g, 0.44 mmol), PMDETA (0.10 mL, 0.48 mmol), tBA (20 mL, 138 mmol) and toluene (12 mL) were sequentially introduced into a 100 mL ampule. The reaction mixture was degassed by three freeze–pump–thaw cycles and purged with nitrogen, and immerged into an oil bath at 80 °C for 5.0 h. After the polymerization was stopped by dipping into liquid nitrogen, the crude product was diluted with THF and passed through a column chromatograph filled with neutral alumina to remove the copper complex, and precipitated into the mixed solvents of methanol and water (Vmethanol/Vwater = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The precipitate was collected and dried at 45 °C in vacuum to a constant weight. GPC: Mn,GPC = 9000 g mol−1, PDI = 1.10. 1H NMR (CDCl3, δ, ppm, TMS): 0.12–0.22 ((CH3)3Si–), 0.78–0.98 (–C(CH3)2–), 1.13–2.48 (15H, aliphatic main chain –CH2CH– on PS chain, aliphatic main chain –CH2CH– on PtBA chain and tert-butyl group protons –C(CH3)3), 3.70–3.90 (BrCHCOO–), 3.95–4.20 (–C
1). The precipitate was collected and dried at 45 °C in vacuum to a constant weight. GPC: Mn,GPC = 9000 g mol−1, PDI = 1.10. 1H NMR (CDCl3, δ, ppm, TMS): 0.12–0.22 ((CH3)3Si–), 0.78–0.98 (–C(CH3)2–), 1.13–2.48 (15H, aliphatic main chain –CH2CH– on PS chain, aliphatic main chain –CH2CH– on PtBA chain and tert-butyl group protons –C(CH3)3), 3.70–3.90 (BrCHCOO–), 3.95–4.20 (–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–CH2O–), 6.30–7.30 (5H, aromatic –C6H5 on PS chain). Mn,NMR = 10
C–CH2O–), 6.30–7.30 (5H, aromatic –C6H5 on PS chain). Mn,NMR = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 g mol−1.
300 g mol−1.![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –PS-b-PtBA-TEMPO) was achieved by NRC reaction between TMS–
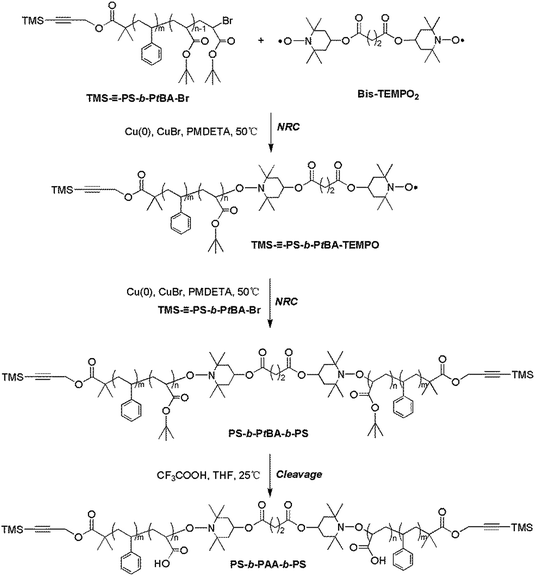
–PS-b-PtBA-TEMPO) was achieved by NRC reaction between TMS–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –PS-b-PtBA-Br and Bis-TEMPO2 (Scheme 3). Into a 100 mL ampule, the TMS-PS-b-PtBA-Br (0.1540 g, 0.017 mmol), Bis-TEMPO2 (0.5090 g, 0.8 mmol), Cu (0.0480 g, 0.75 mmol), CuBr (0.0881 g, 0.60 mmol), Me6TREN (0.2740 g, 1.19 mmol), DMSO (1.5 mL) and toluene (1.5 mL) were introduced successively. The reaction mixture was degassed by three freeze–pump–thaw cycles and purged with nitrogen, and then immerged into an oil bath at 50 °C for 36 h. After the copper powder was removed by centrifugation, the product was purified according to that of TMS–
–PS-b-PtBA-Br and Bis-TEMPO2 (Scheme 3). Into a 100 mL ampule, the TMS-PS-b-PtBA-Br (0.1540 g, 0.017 mmol), Bis-TEMPO2 (0.5090 g, 0.8 mmol), Cu (0.0480 g, 0.75 mmol), CuBr (0.0881 g, 0.60 mmol), Me6TREN (0.2740 g, 1.19 mmol), DMSO (1.5 mL) and toluene (1.5 mL) were introduced successively. The reaction mixture was degassed by three freeze–pump–thaw cycles and purged with nitrogen, and then immerged into an oil bath at 50 °C for 36 h. After the copper powder was removed by centrifugation, the product was purified according to that of TMS–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –PS-b-PtBA-Br, and dried at 45 °C in vacuum to a constant weight.
–PS-b-PtBA-Br, and dried at 45 °C in vacuum to a constant weight.
Then, the triblock copolymer PS-b-PtBA-b-PS was obtained by a second NRC reaction between TMS–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –PS-b-PtBA-TEMPO and TMS–
–PS-b-PtBA-TEMPO and TMS–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –PS-b-PtBA-Br. The sample TMS–
–PS-b-PtBA-Br. The sample TMS–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –PS-b-PtBA-TEMPO (0.1511 g, 0.017 mmol), TMS–
–PS-b-PtBA-TEMPO (0.1511 g, 0.017 mmol), TMS–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –PS-b-PtBA (0.1870 g, 0.02 mmol), Cu(0) (0.0100 g, 0.16 mmol), CuBr (0.0321 g, 0.22 mmol), Me6TREN (0.079 g, 0.34 mmol), DMSO (1.0 mL) and toluene (1.0 mL) were introduced into a 100 mL ampule successively. The reaction mixture was degassed by three freeze–pump–thaw cycles and purged with nitrogen, and then immerged into an oil bath at 50 °C for 36 h. The subsequent purification procedure was also similar to that of TMS–
–PS-b-PtBA (0.1870 g, 0.02 mmol), Cu(0) (0.0100 g, 0.16 mmol), CuBr (0.0321 g, 0.22 mmol), Me6TREN (0.079 g, 0.34 mmol), DMSO (1.0 mL) and toluene (1.0 mL) were introduced into a 100 mL ampule successively. The reaction mixture was degassed by three freeze–pump–thaw cycles and purged with nitrogen, and then immerged into an oil bath at 50 °C for 36 h. The subsequent purification procedure was also similar to that of TMS–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –PS-b-PtBA-Br. In order to remove the uncoupled diblock copolymers, the product was further purified by fractional precipitation from THF/H2O system, and the obtained product was dried at 45 °C in vacuum to a constant weight. GPC: Mn,GPC = 19
–PS-b-PtBA-Br. In order to remove the uncoupled diblock copolymers, the product was further purified by fractional precipitation from THF/H2O system, and the obtained product was dried at 45 °C in vacuum to a constant weight. GPC: Mn,GPC = 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 g mol−1, PDI = 1.13. 1H NMR (CDCl3, δ, ppm, TMS): 2.10–2.48 (–CH2CH– on PtBA chain), 6.30–7.30 (5H, aromatic –C6H5 on PS chain).
800 g mol−1, PDI = 1.13. 1H NMR (CDCl3, δ, ppm, TMS): 2.10–2.48 (–CH2CH– on PtBA chain), 6.30–7.30 (5H, aromatic –C6H5 on PS chain).
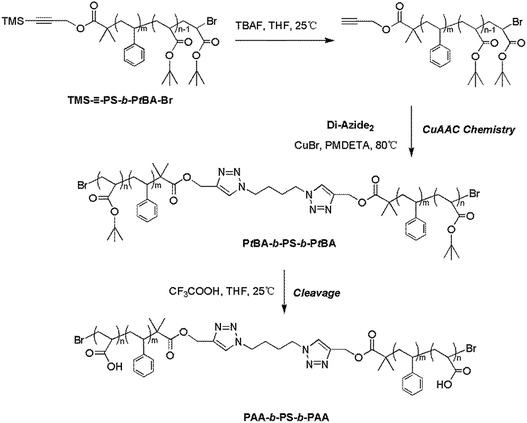
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –PS-b-PtBA-Br (Scheme 4). Typically, the sample TMS–
–PS-b-PtBA-Br (Scheme 4). Typically, the sample TMS–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –PS-b-PtBA-Br (0.7801 g, 0.060 mmol) was dissolved in THF (30 mL) and TBAF (0.29 g, 0.92 mmol) was introduced. After the mixture was immerged into an oil bath at 25 °C for 24 h, the product was precipitated into a mixed solvent of methanol and water (Vmethanol/Vwater = 1
–PS-b-PtBA-Br (0.7801 g, 0.060 mmol) was dissolved in THF (30 mL) and TBAF (0.29 g, 0.92 mmol) was introduced. After the mixture was immerged into an oil bath at 25 °C for 24 h, the product was precipitated into a mixed solvent of methanol and water (Vmethanol/Vwater = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The precipitate was collected and dried at 45 °C in vacuum to a constant weight.
1). The precipitate was collected and dried at 45 °C in vacuum to a constant weight.
Subsequently, the triblock copolymer PtBA-b-PS-b-PtBA was obtained by CuAAC chemistry between Di-Azide2 and Alkynyl-PS-b-PtBA. Alkynyl-PS-b-PtBA (0.2900 g, 0.023 mmol), CuBr (0.0851 g, 0.060 mmol), PMDETA (1.2 mL, 5.76 mmol) and DMF (1.5 mL) were introduced into a 100 mL ampule successively. After the reaction mixture was degassed by three freeze–pump–thaw cycles and purged with nitrogen, the ampule was immerged into an oil bath at 80 °C and Di-Azide2 (0.0231 g, 0.118 mmol) was added into the ampule dropwise by syringe in 12 h. After another 24 h, the crude polymers were recovered by the similar procedure for TMS–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –PS-b-PtBA-Br. The uncoupled diblock copolymers were also removed by fractional precipitation from THF/H2O system, and the obtained product was dried at 45 °C in vacuum to a constant weight. GPC: Mn,GPC = 20
–PS-b-PtBA-Br. The uncoupled diblock copolymers were also removed by fractional precipitation from THF/H2O system, and the obtained product was dried at 45 °C in vacuum to a constant weight. GPC: Mn,GPC = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, PDI = 1.10. 1H NMR (CDCl3, δ, ppm, TMS): 2.10–2.48 (–CH2CH– on PtBA chain), 6.30–7.30 (5H, aromatic –C6H5 on PS chain).
000 g mol−1, PDI = 1.10. 1H NMR (CDCl3, δ, ppm, TMS): 2.10–2.48 (–CH2CH– on PtBA chain), 6.30–7.30 (5H, aromatic –C6H5 on PS chain).
Results and discussion
Synthesis and characterization of diblock copolymer TMS–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/h3_char_e002.gif) –PS-b-PtBA-Br
–PS-b-PtBA-Br
The diblock copolymer TMS–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –PS-b-PtBA-Br with a bromide group and a protected alkynyl end group was prepared by ATRP of tBA monomers from TMS–
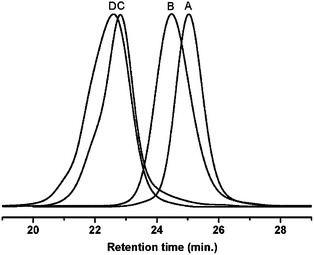
–PS-b-PtBA-Br with a bromide group and a protected alkynyl end group was prepared by ATRP of tBA monomers from TMS–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –PS-Br macro-initiator, which was synthesized by ATRP of St monomers from TMS-PgBiB initiator. Fig. 1A and 1B showed the GPC traces of TMS–
–PS-Br macro-initiator, which was synthesized by ATRP of St monomers from TMS-PgBiB initiator. Fig. 1A and 1B showed the GPC traces of TMS–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –PS-Br and TMS–
–PS-Br and TMS–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –PS-b-PtBA-Br, respectively, the monomodal peaks, low PDIs and the clear shift from TMS–
–PS-b-PtBA-Br, respectively, the monomodal peaks, low PDIs and the clear shift from TMS–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –PS-Br curve to TMS–
–PS-Br curve to TMS–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –PS-b-PtBA-Br curve confirmed that both ATRP procedures were successful.
–PS-b-PtBA-Br curve confirmed that both ATRP procedures were successful.
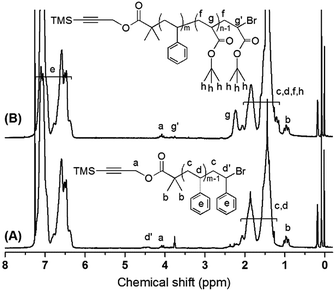
In Fig. 2A for the 1H NMR of TMS–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –PS-Br, except for the characteristic resonance signals for aromatic protons (–C6H5) on PS chain at 6.30–7.30 ppm (e), the 1H NMR spectrum also exhibited the characteristic resonance signals due to methine group proton (BrCHC6H5–) at 4.35–4.55 ppm (d′), propargyl group protons (–C
–PS-Br, except for the characteristic resonance signals for aromatic protons (–C6H5) on PS chain at 6.30–7.30 ppm (e), the 1H NMR spectrum also exhibited the characteristic resonance signals due to methine group proton (BrCHC6H5–) at 4.35–4.55 ppm (d′), propargyl group protons (–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–CH2O–) at 3.95–4.20 ppm (a) and methyl group protons (–C(CH3)2–) at 0.78–0.98 ppm (b), which further proved the successful preparation of TMS–
C–CH2O–) at 3.95–4.20 ppm (a) and methyl group protons (–C(CH3)2–) at 0.78–0.98 ppm (b), which further proved the successful preparation of TMS–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –PS-Br. According to 1H NMR spectrum, the Mn,NMR of TMS–
–PS-Br. According to 1H NMR spectrum, the Mn,NMR of TMS–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –PS-Br was calculated by Formula (1):
–PS-Br was calculated by Formula (1):
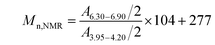 | (1) |
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–CH2O–) at 3.95–4.20 ppm. The values of 104 and 277 were the molecular weight of St unit and that of initiator residue, respectively. The obtained Mn,NMR was 7400 g mol−1, which approximated to that obtained from above GPC measurement (7500 g mol−1). The amount of St unit on each macromolecule was also estimated as 68, and the detailed data was listed in Table 1.
C–CH2O–) at 3.95–4.20 ppm. The values of 104 and 277 were the molecular weight of St unit and that of initiator residue, respectively. The obtained Mn,NMR was 7400 g mol−1, which approximated to that obtained from above GPC measurement (7500 g mol−1). The amount of St unit on each macromolecule was also estimated as 68, and the detailed data was listed in Table 1.
| Entry | Mn,GPCa (g mol−1) | PDIa | Mn,NMR (g mol−1) | NStd | NtBAe |
|---|---|---|---|---|---|
a Determined by GPC performed in THF solvent using PS as standard.b The molecular weight was calculated according to Formula (1).c The molecular weight was calculated according to Formula (2).d The number of St unit was calculated using Formula: NSt = Mn,NMR,(TMS–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –PS-Br)/104, where the value of 104 corresponded to the molecular weight of St unit.e The number of tBA unit was calculated using Formula: NtBA = (Mn,NMR,(TMS– –PS-Br)/104, where the value of 104 corresponded to the molecular weight of St unit.e The number of tBA unit was calculated using Formula: NtBA = (Mn,NMR,(TMS–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –PS-b-PtBA-Br) − Mn,NMR,(TMS– –PS-b-PtBA-Br) − Mn,NMR,(TMS–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –PS-Br))/128, where the value of 128 corresponded to the molecular weight of tBA unit. –PS-Br))/128, where the value of 128 corresponded to the molecular weight of tBA unit. |
|||||
TMS–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –PS-Br –PS-Br |
7500 | 1.06 | 7400b | 68 | |
TMS–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –PS-b-PtBA-Br –PS-b-PtBA-Br |
9000 | 1.10 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300c 300c |
68 | 32 |
| PS-b-PtBA-b-PS | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
1.13 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
136 | 64 |
| PtBA-b-PS-b-PtBA | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.10 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
136 | 64 |
Also, the 1H NMR spectrum of TMS–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –PS-b-PtBA-Br was shown in Fig. 2B, compared with that of TMS–
–PS-b-PtBA-Br was shown in Fig. 2B, compared with that of TMS–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –PS-Br, the new resonance signal at 3.70–3.90 ppm (g′) for methine group proton (BrCHCOO–) and the resonance signal at 2.10–2.48 ppm (g) for methine group proton (–CH2CH) confirmed the successful ATRP of tBA monomers from TMS–
–PS-Br, the new resonance signal at 3.70–3.90 ppm (g′) for methine group proton (BrCHCOO–) and the resonance signal at 2.10–2.48 ppm (g) for methine group proton (–CH2CH) confirmed the successful ATRP of tBA monomers from TMS–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –PS-Br macro-initiator. According to 1H NMR spectrum, the Mn,NMR of TMS–
–PS-Br macro-initiator. According to 1H NMR spectrum, the Mn,NMR of TMS–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –PS-b-PtBA-Br could be derived from Formula (2):
–PS-b-PtBA-Br could be derived from Formula (2):
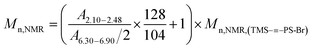 | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 g mol−1, and the amount of PtBA units introduced on each macromolecule was calculated as 23.
300 g mol−1, and the amount of PtBA units introduced on each macromolecule was calculated as 23.
Synthesis and characterization of ABA type triblock copolymer PS-b-PAA-b-PS
For the target ABA type triblock copolymer PS-b-PAA-b-PS, the atom transfer radical coupling (ATRC)40 was initially adopted to synthesize PS-b-PtBA-b-PS. However, almost no coupling product was discriminated from GPC measurement, which can be ascribed to the disproportionation termination on terminal PtBA-Br.41–43 Especially, the higher molecular weight of precursor would bring a larger hindrance, and further led to a lower coupling efficiency. Alternatively, the NRC reaction was considered to synthesize PS-b-PAA-b-PS. Firstly, its precursor PS-b-PtBA-b-PS was obtained by NRC reaction between TMS–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –PS-b-PtBA-Br and TMS–
–PS-b-PtBA-Br and TMS–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –PS-b-PtBA-TEMPO, and the latter was first prepared by NRC reaction between TMS–
–PS-b-PtBA-TEMPO, and the latter was first prepared by NRC reaction between TMS–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –PS-b-PtBA-Br and the coupling agent Bis-TEMPO2.
–PS-b-PtBA-Br and the coupling agent Bis-TEMPO2.
In order to obtain a high coupling efficiency, the feed procedure was well analyzed and balanced. In the first case, an attempt was made to couple the TMS–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –PS-b-PtBA-Br and Bis-TEMPO2 directly to get the target PS-b-PtBA-b-PS. That was, the TMS–
–PS-b-PtBA-Br and Bis-TEMPO2 directly to get the target PS-b-PtBA-b-PS. That was, the TMS–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –PS-b-PtBA-Br and Bis-TEMPO2 were simultaneously added and sealed in the system. However, the inevitable equivalent deviation between the macromolecule TMS–
–PS-b-PtBA-Br and Bis-TEMPO2 were simultaneously added and sealed in the system. However, the inevitable equivalent deviation between the macromolecule TMS–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –PS-b-PtBA-Br and small molecular weight compound Bis-TEMPO2 always produced amount of uncoupled precursors. Alternatively, the equivalent deviation between macromolecules and Bis-TEMPO2 might be solved by a dropwise addition procedure. Thus, in the second case, the macromolecule TMS–
–PS-b-PtBA-Br and small molecular weight compound Bis-TEMPO2 always produced amount of uncoupled precursors. Alternatively, the equivalent deviation between macromolecules and Bis-TEMPO2 might be solved by a dropwise addition procedure. Thus, in the second case, the macromolecule TMS–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –PS-b-PtBA-Br was first added and the compound Bis-TEMPO2 was added dropwise by syringe. However, the trace oxygen might be introduced by syringe, which would have a vital effect on the NRC reaction. Also, the instantaneous low concentration of TEMPO groups in system would not guarantee the sufficient NRC reaction and the side reaction (such as the disproportionation termination) of generated carbon radicals might be enhanced. Conversely, in the third case, the compound Bis-TEMPO2 was first added and the macromolecule TMS–
–PS-b-PtBA-Br was first added and the compound Bis-TEMPO2 was added dropwise by syringe. However, the trace oxygen might be introduced by syringe, which would have a vital effect on the NRC reaction. Also, the instantaneous low concentration of TEMPO groups in system would not guarantee the sufficient NRC reaction and the side reaction (such as the disproportionation termination) of generated carbon radicals might be enhanced. Conversely, in the third case, the compound Bis-TEMPO2 was first added and the macromolecule TMS–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –PS-b-PtBA-Br in toluene was then added dropwise. Similarly, except for the introduction of trace oxygen, the viscous TMS–
–PS-b-PtBA-Br in toluene was then added dropwise. Similarly, except for the introduction of trace oxygen, the viscous TMS–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –PS-b-PtBA-Br solution also increased the trouble of feeding procedure. Thus, in order to avoid the equivalent deviation from calculation and the introduction of trace oxygen, as well as reduce the side reaction of formed carbon radical in system and simplify the operation, a fourth route in this work was designed and adopted. That was, the precursor TMS–
–PS-b-PtBA-Br solution also increased the trouble of feeding procedure. Thus, in order to avoid the equivalent deviation from calculation and the introduction of trace oxygen, as well as reduce the side reaction of formed carbon radical in system and simplify the operation, a fourth route in this work was designed and adopted. That was, the precursor TMS–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –PS-b-PtBA-TEMPO was first formed by NRC reaction between TMS–
–PS-b-PtBA-TEMPO was first formed by NRC reaction between TMS–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –PS-b-PtBA-Br and excess coupling agent Bis-TEMPO2, and the purified TMS–
–PS-b-PtBA-Br and excess coupling agent Bis-TEMPO2, and the purified TMS–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –PS-b-PtBA-TEMPO (see Fig. S1 in ESI†) could then couple with the precursor TMS–
–PS-b-PtBA-TEMPO (see Fig. S1 in ESI†) could then couple with the precursor TMS–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –PS-b-PtBA-Br.
–PS-b-PtBA-Br.
After the purification of crude product, Fig. 1C showed the GPC trace of PS-b-PtBA-b-PS. The monomodal peak, low PDI (1.13) and the significantly increased Mn,GPC (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 g mol−1) compared with that of TMS–
800 g mol−1) compared with that of TMS–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –PS-b-PtBA-Br confirmed that the NRC was successful. In Fig. 3A, except for the characteristic resonance signals for aromatic protons (–C6H5) on PS chain at 6.30–7.30 ppm (a), the 1H NMR spectrum of PS-b-PtBA-b-PS also exhibited the characteristic resonance signal due to methine group proton (–CH2CH–) at 2.10–2.48 ppm (e). Finally, the ABA type triblock copolymer PS-b-PAA-b-PS was further prepared by cleavage of PtBA segment on PS-b-PtBA-b-PS in THF solvent in the presence of CF3COOH. Fig. 3B showed the 1H NMR spectrum of PS-b-PAA-b-PS in DMF-d7. Compared with the spectrum of PS-b-PtBA-b-PS, the resonance signal at 1.13–2.48 ppm in the spectrum of PS-b-PAA-b-PS was obviously weakened, which showed that the tert-butyl groups on PtBA segment were removed. Thus, all the above information clearly confirmed that the ABA type triblock copolymer PS-b-PAA-b-PS was obtained.
–PS-b-PtBA-Br confirmed that the NRC was successful. In Fig. 3A, except for the characteristic resonance signals for aromatic protons (–C6H5) on PS chain at 6.30–7.30 ppm (a), the 1H NMR spectrum of PS-b-PtBA-b-PS also exhibited the characteristic resonance signal due to methine group proton (–CH2CH–) at 2.10–2.48 ppm (e). Finally, the ABA type triblock copolymer PS-b-PAA-b-PS was further prepared by cleavage of PtBA segment on PS-b-PtBA-b-PS in THF solvent in the presence of CF3COOH. Fig. 3B showed the 1H NMR spectrum of PS-b-PAA-b-PS in DMF-d7. Compared with the spectrum of PS-b-PtBA-b-PS, the resonance signal at 1.13–2.48 ppm in the spectrum of PS-b-PAA-b-PS was obviously weakened, which showed that the tert-butyl groups on PtBA segment were removed. Thus, all the above information clearly confirmed that the ABA type triblock copolymer PS-b-PAA-b-PS was obtained.
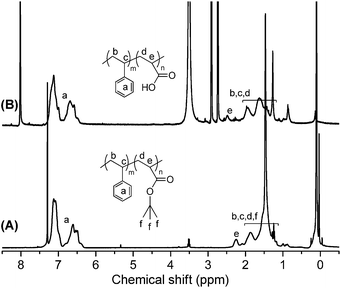 | ||
| Fig. 3 The 1H NMR spectra of PS-b-PtBA-b-PS (A) (in CDCl3 solvent) and PS-b-PAA-b-PS (B) (in DMF-d7 solvent). | ||
Synthesis and characterization of BAB type triblock copolymer PAA-b-PS-b-PAA
Also, in order to get the target BAB type triblock copolymer PAA-b-PS-b-PAA, its precursor PtBA-b-PS-b-PtBA was prepared by CuAAC chemistry between Alkynyl-PS-b-PtBA-Br (see Fig. S2 in ESI†) and the coupling agent Di-Azide2, and the former was derived from the cleavage of protected group TMS–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –PS-b-PtBA-Br in the presence of TBAF. Unlike the NRC reaction, because the CuAAC chemistry tolerates a certain content of oxygen, the dropwise addition procedure could be considered in this system. That was, the Alkynyl-PS-b-PtBA-Br was firstly added and Di-Azide2 was then added dropwise, which could largely exclude the equivalent deviation between macromolecules Alkynyl-PS-b-PtBA-Br and small molecular weight compound Di-Azide2 and further ensure the high coupling efficiency. As mentioned in the above section, the terminal PtBA-Br tended to the disproportionation termination43 and the products formed from ATRC reaction can be neglected. Thus, under this CuAAC chemistry condition, the interruption of ATRC reaction can be excluded and the aimed product PtBA-b-PS-b-PtBA was achieved.
–PS-b-PtBA-Br in the presence of TBAF. Unlike the NRC reaction, because the CuAAC chemistry tolerates a certain content of oxygen, the dropwise addition procedure could be considered in this system. That was, the Alkynyl-PS-b-PtBA-Br was firstly added and Di-Azide2 was then added dropwise, which could largely exclude the equivalent deviation between macromolecules Alkynyl-PS-b-PtBA-Br and small molecular weight compound Di-Azide2 and further ensure the high coupling efficiency. As mentioned in the above section, the terminal PtBA-Br tended to the disproportionation termination43 and the products formed from ATRC reaction can be neglected. Thus, under this CuAAC chemistry condition, the interruption of ATRC reaction can be excluded and the aimed product PtBA-b-PS-b-PtBA was achieved.
Fig. 1D showed the GPC trace of purified PtBA-b-PS-b-PtBA. The monomodal peak, low PDI (1.10), as well as the increased Mn,GPC (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) confirmed that the CuAAC chemistry was successful. The 1H NMR spectra of PtBA-b-PS-b-PtBA and PAA-b-PS-b-PAA showed similar results to those of the above PS-b-PtBA-b-PS and PS-b-PAA-b-PS (see Fig. S3 in ESI†), which further meant that these two series of triblock copolymers actually had the identical compositions but different block sequences.
000 g mol−1) confirmed that the CuAAC chemistry was successful. The 1H NMR spectra of PtBA-b-PS-b-PtBA and PAA-b-PS-b-PAA showed similar results to those of the above PS-b-PtBA-b-PS and PS-b-PAA-b-PS (see Fig. S3 in ESI†), which further meant that these two series of triblock copolymers actually had the identical compositions but different block sequences.
Self-assembly behaviour of amphiphilic ABA type PS-b-PAA-b-PS and BAB type PAA-b-PS-b-PAA triblock copolymer
The amphiphilic nature of triblock copolymer PS-b-PAA-b-PS or PAA-b-PS-b-PAA, consisting of hydrophobic PS chain and hydrophilic PAA chain, provided an opportunity to form micelles in a selective solvent (such as water). The cmc of triblock copolymer in water was determined by fluorescence technique using PNA as probe, which is a more suitable fluorescent probe than pyrene in terms of reproducibility because it displays higher fluorescence activity in nonpolar environments and can also be very easily quenched by polar solvents (such as water).39 The relationship of the fluorescence intensity ratio (I/I0) of PNA as a function of the concentration of PS-b-PAA-b-PS was plotted in Fig. 4A. It was found that I/I0 increased sharply when the concentration exceeded a certain value, which demonstrated that the PNA probe began to be incorporated into the hydrophobic region of micelles. Thus, the intersection of two straight lines with a value of 8.44 × 10−3 g L−1 was determined as the cmc of PS-b-PAA-b-PS. Similarly, the cmc of PAA-b-PS-b-PAA was also determined as 2.62 × 10−2 g L−1 according to Fig. 4B. Obviously, the cmc value of PS-b-PAA-b-PS was about 32 times higher than that of PAA-b-PS-b-PAA. In a parallel level, with the identical compositions but different block sequences, these two copolymers show distinct cmc values. This result could give the information that the block sequence (connection style of segment) actually has effect on the self-assembly behaviour of amphiphilic triblock copolymers.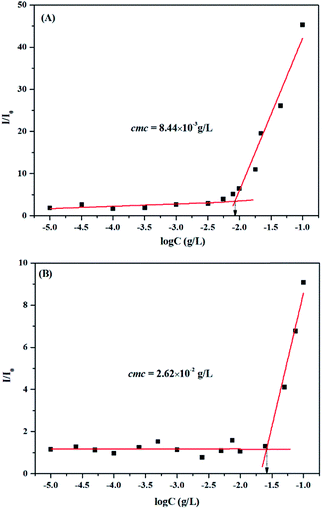 | ||
| Fig. 4 Dependence of fluorescence intensity ratio I/I0 of PNA fluorescence emission spectra on the concentration of PS-b-PAA-b-PS (A) or PAA-b-PS-b-PAA (B) triblock copolymer (λex = 340 nm). | ||
To further investigate the differences of solution behaviour between the triblock copolymer PS-b-PAA-b-PS and PAA-b-PS-b-PAA, the micelle morphology was observed by FESEM instrument. As a reference, the micelle morphology of PS-b-PAA was also observed simultaneously. The diblock copolymer PS-b-PAA was obtained by the typical cleavage of PtBA segment on TMS–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –PS-b-PtBA-Br by CF3COOH. Fig. 5A–C showed the micelle morphologies of PS-b-PAA, PS-b-PAA-b-PS and PAA-b-PS-b-PAA in water, respectively. Small particles could be found scattered in all the three FESEM images, which meant that the micelles were formed by all the three copolymers. Differently, it was found that the size of micelles from triblock copolymer PS-b-PAA-b-PS was similar to the that of micelles from diblock copolymer PS-b-PAA, while the size of micelles from triblock copolymer PAA-b-PS-b-PAA were somewhat lager. That was, the average diameter of PS-b-PAA micelles or PS-b-PAA-b-PS micelles was around 30 nm, and the average diameter of PAA-b-PS-b-PAA micelles was around 47 nm. Also, by DLS measurement, the sizes of micelles formed by PS-b-PAA, PS-b-PAA-b-PS and PAA-b-PS-b-PAA copolymers were obtained as 17 nm, 18 nm and 44 nm, respectively (Fig. S4†), which were well agreed with those by FESEM instrument.
–PS-b-PtBA-Br by CF3COOH. Fig. 5A–C showed the micelle morphologies of PS-b-PAA, PS-b-PAA-b-PS and PAA-b-PS-b-PAA in water, respectively. Small particles could be found scattered in all the three FESEM images, which meant that the micelles were formed by all the three copolymers. Differently, it was found that the size of micelles from triblock copolymer PS-b-PAA-b-PS was similar to the that of micelles from diblock copolymer PS-b-PAA, while the size of micelles from triblock copolymer PAA-b-PS-b-PAA were somewhat lager. That was, the average diameter of PS-b-PAA micelles or PS-b-PAA-b-PS micelles was around 30 nm, and the average diameter of PAA-b-PS-b-PAA micelles was around 47 nm. Also, by DLS measurement, the sizes of micelles formed by PS-b-PAA, PS-b-PAA-b-PS and PAA-b-PS-b-PAA copolymers were obtained as 17 nm, 18 nm and 44 nm, respectively (Fig. S4†), which were well agreed with those by FESEM instrument.
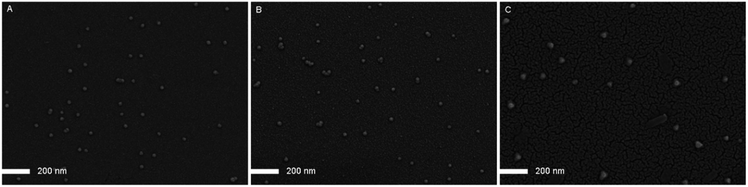 | ||
| Fig. 5 FESEM images of micelles formed by PS-b-PAA (A), PS-b-PAA-b-PS (B) and PAA-b-PS-b-PAA (C) in water. | ||
From Fig. 5A–C, all these amphiphilic copolymers showed the classical spherical morphologies in water. As has been well investigated, the spherical morphologies were the simplest morphologies of micelles, in which the hydrophobic segments tend to form a core inside the micelle while the hydrophilic segments tend to form a shell outside the micelle (Scheme 5).44 Based on the result that the size of PS-b-PAA-b-PS micelles was similar to PS-b-PAA micelles, while the PAA-b-PS-b-PAA micelles were somewhat larger, we might figure that the block sequence of the copolymers played a very important role in the formation of micelles. For triblock copolymer PS-b-PAA-b-PS, because both ends of PAA segment were seriously restricted by PS segments, the outer PAA shell in micelle could not be well solubilized in water. Differently, for triblock copolymer PAA-b-PS-b-PAA, only one end of PAA segment was connected on PS segment, and the outer PAA segments in micelle could wind into water freely. This fact could also explain why the size of PAA-b-PS-b-PAA micelles were larger than that of PS-b-PAA-b-PS micelles.
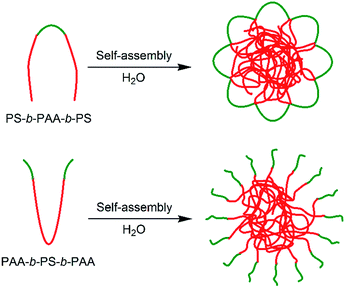 | ||
| Scheme 5 The proposed mechanism for the formation of micelles of triblock copolymer PS-b-PAA-b-PS and PAA-b-PS-b-PAA with identical compositions but different block sequences in selective solvent. | ||
As described in Introduction section, although plenty of works had focused on the self-assembly behaviour of amphiphilic polymers, rare of them had aimed to study the effect of solvents, compositions, topologies on their properties changing a single variable at once. In this contribution, based on the precisely designed synthetic route, the ABA type triblock copolymer PS-b-PAA-b-PS and BAB type triblock copolymer PAA-b-PS-b-PAA were both synthesized from the same precursor of TMS–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –PS-b-PtBA-Br, which made the two triblock copolymers share the identical compositions but different block sequences. The different self-assembly behaviour of these copolymers in water might be an important result to enrich the structure–property relationship. Also, this difference might be evidence that the triblock copolymers with identical compositions but different block sequences were actually synthesized.
–PS-b-PtBA-Br, which made the two triblock copolymers share the identical compositions but different block sequences. The different self-assembly behaviour of these copolymers in water might be an important result to enrich the structure–property relationship. Also, this difference might be evidence that the triblock copolymers with identical compositions but different block sequences were actually synthesized.
Conclusions
In conclusion, the amphiphilic ABA type PS-b-PAA-b-PS and BAB type PAA-b-PS-b-PAA triblock copolymers with identical compositions but different block sequences were successfully synthesized by combination of ATRP mechanism and NRC or CuAAC chemistry. This versatile synthetic route might be used to derive series of copolymers with varied compositions. The self-assembly behaviour of copolymers was preliminarily studied and compared, and the results showed that the PS-b-PAA-b-PS and PAA-b-PS-b-PAA could give distinct cmc values and different sizes of micelles in selective solvent, which further enriches the related theory of structure–property relationship.Acknowledgements
We appreciate the financial support of this research by the Natural Science Foundation of China (21274024).Notes and references
- W. J. Lin, W. C. Chen, W. C. Wu, Y. H. Niu and A. K. Y. Jen, Macromolecules, 2004, 37, 2335–2341 CrossRef CAS.
- R. Yoshida, K. Uchida, Y. Kaneko, K. Sakai, A. Kikuchi, Y. Sakurai and T. Okano, Nature, 1995, 374, 240–242 CrossRef CAS.
- B. L. Liu, A. Kazlauciunas, J. T. Guthrie and S. Perrier, Macromolecules, 2005, 38, 2131–2136 CrossRef CAS.
- M. Jikei and M. Kakimoto, Prog. Polym. Sci., 2001, 26, 1233–1285 CrossRef CAS.
- Y. Zhang, G. Wang and J. Huang, Macromolecules, 2010, 43, 10343–10347 CrossRef CAS.
- Z. Jia, Q. Fu and J. Huang, Macromolecules, 2006, 39, 5190–5193 CrossRef CAS.
- Y. Yagci and M. A. Tasdelen, Prog. Polym. Sci., 2006, 31, 1133–1170 CrossRef CAS PubMed.
- L. F. Zhang and A. Eisenberg, J. Am. Chem. Soc., 1996, 118, 3168–3181 CrossRef CAS.
- L. F. Zhang and A. Eisenberg, Science, 1995, 268, 1728–1731 CAS.
- Z. S. Gao, S. K. Varshney, S. Wong and A. Eisenberg, Macromolecules, 1994, 27, 7923–7927 CrossRef CAS.
- L. F. Zhang, K. Yu and A. Eisenberg, Science, 1996, 272, 1777–1779 CAS.
- L. F. Zhang and A. Eisenberg, Polym. Adv. Technol., 1998, 9, 677–699 CrossRef CAS.
- L. F. Zhang and A. Eisenberg, Macromolecules, 1999, 32, 2239–2249 CrossRef CAS.
- H. W. Shen and A. Eisenberg, Macromolecules, 2000, 33, 2561–2572 CrossRef CAS.
- H. Liang, B. D. Favis, Y. S. Yu and A. Eisenberg, Macromolecules, 1999, 32, 1637–1642 CrossRef CAS.
- L. F. Zhang and A. Eisenberg, Macromolecules, 1996, 29, 8805–8815 CrossRef CAS.
- H. W. Shen and A. Eisenberg, Angew. Chem., Int. Ed., 2000, 39, 3310–3312 CrossRef CAS.
- L. F. Zhang, C. Bartels, Y. S. Yu, H. W. Shen and A. Eisenberg, Phys. Rev. Lett., 1997, 79, 5034–5037 CrossRef CAS.
- J. Ruehl, A. Nilsen, S. Born, P. Thoniyot, L.-P. Xu, S. Chen and R. Braslau, Polymer, 2007, 48, 2564–2571 CrossRef CAS PubMed.
- Y. Zhang, W. Lin, R. Jing and J. Huang, J. Phys. Chem. B, 2008, 112, 16455–16460 CrossRef CAS.
- H. Hückstädt, A. Göpfert and V. Abetz, Polymer, 2000, 41, 9089–9094 CrossRef.
- Z. Chen, H. Cui, K. Hales, Z. Li, K. Qi, D. J. Pochan and K. L. Wooley, J. Am. Chem. Soc., 2005, 127, 8592–8593 CrossRef CAS PubMed.
- T. Azzam and A. Eisenberg, Angew. Chem., Int. Ed., 2006, 45, 7443–7447 CrossRef PubMed.
- A. H. El-Sagheer and T. Brown, Chem. Soc. Rev., 2010, 39, 1388–1405 RSC.
- F. Vogtle, S. Gestermann, R. Hesse, H. Schwierz and B. Windisch, Prog. Polym. Sci., 2000, 25, 987–1041 CrossRef CAS.
- M. Murat and G. S. Grest, Macromolecules, 1996, 29, 1278–1285 CrossRef CAS.
- O. A. Matthews, A. N. Shipway and J. F. Stoddart, Prog. Polym. Sci., 1998, 23, 1–56 CrossRef CAS.
- W. H. Binder and R. Sachsenhofer, Macromol. Rapid Commun., 2007, 28, 15–54 CrossRef CAS PubMed.
- Q. Fu, W. Lin and J. Huang, Macromolecules, 2008, 41, 2381–2387 CrossRef CAS.
- Q. Fu, Z. Zhang, W. Lin and J. Huang, Macromolecules, 2009, 42, 4381–4383 CrossRef CAS.
- J. Kulis, C. A. Bell, A. S. Micallef, Z. Jia and M. J. Monteiro, Macromolecules, 2009, 42, 8218–8227 CrossRef CAS.
- K. Zhang, P. Zhuang, Z. Wang, Y. Li, Z. Jiang, Q. Hu, M. Liu and Q. Zhao, Carbohydr. Polym., 2012, 90, 1515–1521 CrossRef CAS PubMed.
- W. Lin, Q. Fu, Y. Zhang and J. Huang, Macromolecules, 2008, 41, 4127–4135 CrossRef CAS.
- Y. Li, Y. Zhang, D. Yang, Y. Li, J. Hu, C. Feng, S. Zhai, G. Lu and X. Huang, Macromolecules, 2010, 43, 262–270 CrossRef CAS.
- J. A. Opsteen and J. C. M. van Hest, Chem. Commun., 2005, 57–59 RSC.
- T. Kurosaki, K. Wanlee and M. Okawara, J. Polym. Sci., Part A: Polym. Chem., 1972, 10, 3295–3310 CrossRef CAS PubMed.
- R. Nicolay, L. Marx, P. Hemery and K. Matyjaszewski, Macromolecules, 2007, 40, 9217–9223 CrossRef CAS.
- X. Fan, B. Huang, G. Wang and J. Huang, Macromolecules, 2012, 45, 3779–3786 CrossRef CAS.
- L. C. You, F. Z. Lu, Z. C. Li, W. Zhang and F. M. Li, Macromolecules, 2003, 36, 1–4 CrossRef CAS.
- C. Yoshikawa, A. Goto and T. Fukuda, e-Polym., 2002, 2, 172–183 Search PubMed.
- M. H. Wrue, A. C. McUmber and M. Anthamatten, Macromolecules, 2009, 42, 9255–9262 CrossRef CAS.
- B. A. Gan and Y. Yagci, Turk. J. Chem., 2007, 31, 1–10 Search PubMed.
- G. Wang and J. Huang, Polym. Chem., 2014, 5, 277–308 RSC.
- A. Halperin, M. Tirrell and T. P. Lodge, Adv. Polym. Sci., 1992, 100, 31–71 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: More information of 1H NMR spectra for intermediates and target copolymers and DLS results. See DOI: 10.1039/c4ra07084d |
| This journal is © The Royal Society of Chemistry 2014 |