Functionalized nanometer-sized alumina supported micro-solid phase extraction coupled to inductively coupled plasma mass spectrometry for preconcentration and determination of trace metal ions in gasoline samples†
Philiswa N. Nomngongo and
J. Catherine Ngila*
Department of Applied Chemistry, Faculty Science, University of Johannesburg, Doornfotein Campus, P.O. Box 17011, 2028, Johannesburg, South Africa. E-mail: jcngila@uj.ac.za; Fax: +27-11559 6425; Tel: +27-115596196
First published on 8th September 2014
Abstract
Nanometer-sized alumina functionalized with [3-(2-aminoethylamino) propyl] trimethoxysilane (nano-Al2O3/AAPTMS) was prepared as an adsorbent for preconcentration of trace element ions in gasoline samples. The nano-Al2O3 was characterized by XRD, SEM and BET techniques and the functionalized adsorbent was characterized by ATR-FTIR spectroscopy. The nano-Al2O3/AAPTMS sorbent was used as the packing material in the supported micro-solid-phase extraction (μ-SPE) device. The latter was coupled with inductively coupled plasma mass spectrometry (ICP-MS) for preconcentration and determination of trace elements in gasoline samples. The optimization of the preconcentration system was achieved by a multivariate strategy. Under optimized conditions, limits of detection (LOD) and quantification (LOQ) ranged from 0.2–0.7 ng L−1 to 0.7–2.3 ng L−1, respectively, and a preconcentration factor of 40 was achieved. The validity of the developed μ-SPE-ICP-MS procedure was confirmed by analysis of spiked gasoline samples. The supported μ-SPE-ICP-MS method was applied for quantification of Co, Cr, Mn, Ni and Ti in commercial gasoline samples. The μSPE device coupled with ICP-MS provided improved LOD and LOQ for trace metal analysis in a gasoline matrix and significantly reduced matrix interference.
1 Introduction
The presence of metal ions in liquid fuel such as gasoline is undesirable, not only due to the possibility of damaging vehicle parts, gum formation, catalytic poisoning and poor fuel performance, but also because of the environmental and atmospheric pollution caused by the release of harmful metals during gasoline combustion.1 Metallic elements are generally present in very low concentrations in fuel samples. Therefore, sensitive detection or sample pretreatment techniques are required.1Direct determination of metal ions in gasoline samples by sensitive instrumental techniques such as inductively coupled plasma mass spectrometry (ICP-MS) is often limited due to high carbon content. For this reason, analysis of gasoline using ICP-MS needs special care in order to reduce the formation of carbon deposits on the cones (sampler and skimmer) and in the ion lens of the mass spectrometer as well as to avoid the instability or even the complete extinction of the plasma due to organic vapor overloading.1–3 In addition, polyatomic interferences caused by carbon–argon species could limit accurate quantification of some trace elements such as Cr, Ni and Ti.3 Therefore, in order to minimize the problem surrounding direct introduction of organics in ICP-MS, special introduction tools such as ultrasonic and microflow nebulizers, have been employed.3 As a result, methods based on direct introduction of organic samples such as crude oil and its derivatives are reported in literature.2–5 Notwithstanding the practices involving direct introduction of samples into ICP-MS, many laboratories that cannot afford special accessories to cater for this, do rely on sample pretreatment techniques. For these reasons, sample introduction techniques, such as electrothermal vaporization, have been developed and are reported in literature.1,6,7 Consequently, development of other alternative sample preparation methods prior to the determination of trace metal ions is required.
Recently, few studies have reported on the extraction and enrichment of trace metals in fuel based samples using simple methods such as solid phase extraction (SPE).8–16 The advantages of solid phase extraction include high sensitivity, simultaneous enrichment and matrix elimination in one step, reduced matrix interferences, easily automated, relatively high enrichment factors, and low cost.15–18 In addition, SPE has become more attractive due to the use of different adsorbent materials with high sorption capacities and it can improve sensitivities (detection limits) of the analytical techniques. In recent years, micro-solid phase extraction (μ-SPE) has been developed as an alternative to the multistep conventional SPE method. The advantages of μ-SPE include reduced solvent consumption, sorbents usage and sample handling. In μ-SPE, the adsorbent is trapped in a porous polypropylene membrane sheet. The porosity of the membrane allows the analyte of interest is able to diffuse freely and extracted by the sorbent.19–22
Metal oxide adsorbents such as alumina, titania and zirconia have been reported for the extraction and enrichment of metal ions.17,23–25 They are attractive as adsorbents due to their large surface area, high mechanical properties, strong resistivity to thermal degradation and ability to chemisorb many substances.26 The use of nano-Al2O3 as SPE sorbent material for preconcentration of trace metals has recently received more attention.17,23,27 Notwithstanding the attractive adsorption properties of nano-Al2O3, the latter has relatively poor sorption efficiency towards some metal ions. Thus, functionalization (modification) of the nano-Al2O3 surface with functional groups that contains oxygen, nitrogen and sulfur among others, becomes a solution.28–30 By functionalizing the metal oxide surface, the metal ion removal mechanism has changed. This implies that the analyte of interest is not only removed by adsorption onto the metal oxide surface, but also by a surface attraction/chemical-bonding phenomenon on the newly functional group.30
Therefore, this study reports on the development of nano-Al2O3/AAPTMS supported μ-SPE technique coupled to ICP-MS for simultaneous preconcentration and determination of trace metals in gasoline samples. Optimization of parameters affecting the extraction and preconcentration process by supported μ-SPE (sample pH, eluent concentration and sample flow rate) was achieved by full two-level factorial design with replicates of the central point.
2 Experimental
2.1 Instrumentation
Perkin Elmer NexION 300 ICP-MS Spectrometer with a The NexION 300 (PerkinElmer, Shelton, CT) inductively coupled plasma mass spectrometer (ICP-MS) equipped with triple cone interface, two modes of operation (collision and standard modes) and a single quadrupole was used throughout the analysis, was used for all measurements. The ICP-MS instrument was optimized daily and operated as recommended by the manufacturer. It should be noted that all elements in the samples were measured with kinetic energy discrimination (KED) mode using helium as the collision gas. The collision mode is known to eliminate many argon based polyatomic spectral interferences. The operating conditions are presented in Table 1. Argon of 99.996% purity (Afrox, South Africa) was used.| Component/parameter | Type/value/mode |
|---|---|
| a IS = Internal standard. | |
| Nebulizer | Glass concentric |
| Spray | Chamber glass cyclonic |
| Cones | Nickel |
| Plasma gas flow | 18 L min |
| Auxiliary gas flow | 1.2 L min |
| Nebulizer gas flow | 0.98 L min |
| Sample uptake rate | 300 L min |
| RF power | 1600 W |
| Total intergration time | 0.5 s |
| No. of replicates | 3 |
| Mode of operation | KED |
| Isotopes | 59Co, 52Cr, 55Mn, 60Ni and 48Ti, 45Sc (ISa) |
Morphological structure of the Al2O3 was observed using scanning electron microscope (SEM, VEGA3, TESCAN, Czech Republic) after carbon coating. The specific surface area of nano-Al2O3 was determined by Surface Area and Porosity Analyzer (ASAP2020 V3.00H, Micromeritics Instrument Corporation, Norcross, USA). All the gases used for analysis were instrument grade. X-ray powder diffraction (XRD) measurements were carried out with a Philips X-ray generator model PW 3710/31 a diffractometer with automatic sample changer model PW 1775 (scintillation counter, Cu-target tube and Ni-filter at 40 kV and 30 mA). Infrared spectrum was recorded using Spectrum 100 FT-IR (PerkinElmer, USA) spectrometer equipped with Universal Attenuated Total Reflectance (ATR).
2.2 Reagents, solutions and materials
All reagents were of analytical grade unless otherwise stated and ultrapure water (specific conductance 0.05 μs cm−1) from a Millipore Waters Milli Q purification unit (Merck Millipore, Bedford, MA, USA) was used throughout the experiments. Anhydrous aluminium chloride (Sigma-Aldrich, St. Loius, MO, USA) was used as a precursor for the preparation of nanometer-sized alumina (nano-Al2O3). Synthetic gasoline was prepared by mixing 91% isooctane and 9% n-heptane (Sigma-Aldrich). Conostan single element oil standard (1.0 mg L−1) of Co, Cr, Mn, Ni, and Ti, (SCP Science, Quebec, Canada) were used to prepare the working solutions for SPE at concentrations of 10 μg L−1 for other metal ions. A Spectrascan multi-element standard solution at a concentration of 100 mg L−1 (Teknolab, Norway) was used to prepare working standard solutions for quantification of analyte concentrations in model and sample solutions. Scandium was used for internal standardization. Nitric acid solutions were prepared from ultrapure concentrated acid (65%, Sigma-Aldrich). The sample pH was adjusted using dilute glacial acetic acid (Merck http://www.merck.com/, Darmstadt, Germany) and ammonia (Sigma-Aldrich) solutions. [3-(2-Aminoethylamino) propyl] trimethoxysilane (AAPTMS), ethanol and toluene were purchased from Sigma Aldrich. The Accurel S6/2 porous polypropylene hollow fiber membrane (Membrana, Wuppertal, Germany) with 0.2 μm pore size, wall thickness of 450 μm and the inner diameter of 1800 μm, was used for the preparation of the μ-SPE.2.3 Preparation of nanometer-sized alumina using sol–gel method
Nano-Al2O3 was prepared according to Rogojan et al.31 A mass (2.66 g) of AlCl3 was dissolved in 25 mL absolute ethanol followed by drop wise addition of 28% ammonium solution. The addition of the latter was done in order to for a sol–gel to form. The resulting sol–gel was left to maturate for 30 hours at room temperature and then dried for 24 hours at 100 °C. Finally, the gel was calcined by heating in a furnace at a rate of 20 °C min−1 to 1000 °C and holding it for three hours.2.4 Functionalization of nanometer-sized alumina with AAPTMS and preparation of supported μ-SPE device
Before functionalization, the nano-Al2O3 was first activated according to the procedure reported by Hang et al.32 About 30 g of nano-Al2O3 was place in 5 mol−1 HNO3 and refluxed for 6 h at 60 °C with constant stirring with a magnetic stirrer. Afterwards, the activated nano-Al2O3 was filtered through a 0.45 μm membrane and washed with ultrapure water and ethanol several times and dried in vacuum. The functionalization nano-Al2O3 with AAPTMS was carried out according to the procedure reported by Tarley et al.33 Briefly, about 10 g of nano-Al2O3 was mixed 6 mL of AAPMTS in toluene (solvent) (20 mL). The reaction was heated at 100 °C for 24 h under nitrogen atmosphere and it was stirred continuously using a heater stirrer. Subsequently, the mixture was filtered and washed with toluene and ethanol. The final product (nano-Al2O3) was washed with ethanol in a Soxhlet extractor for 6 h and then dried at 60 °C for 4 h under vacuum.2.5 Preparation of supported μ-SPE device and flow preconcentration system
The preparation of the μ-SPE device was carried out according to previous studies with some modification.19,22 Briefly, the μ-SPE device contained nano-Al2O3/AAPMTS solid material enclosed within a polypropylene hollow fiber membrane. The hollow fiber membrane was cut into 6 cm segments. One of the two open ends of each segment was heat-sealed using an electrical sealer. A Pasteur pipette was used to introduce a slurried sorbent (50 mg) via the remaining open end of hollow fiber. The latter was then heat-sealed to secure the content. Before use, each device was cleaned by ultrasonication in ultrapure water (2 min) and diluted nitric acid (2 min). The μ-SPE device was the stored in ultrapure water until its use.The Minipuls™ 3 peristaltic pumps (Gilson, Villiers le Bel, France) were used for the flow system. A self-made PTFE micro-column (6.0 cm, 2.85 mm i.d), packed with μ-SPE device was used in the manifold for extraction and preconcentration of the target analytes. Solvent Flex and PVC peristaltic pump tubing (Black/Black 0.76 mm i.d) were employed to propel the sample/buffer and eluent, respectively. Minimum lengths of PTFE tubing were used for all connections.
2.6 General procedure
Due to the absence of blank gasoline solution, synthetic gasoline (prepared by mixing 91% isooctane and 9% n-heptane) was prepared and used as a model solution. It should be noted that synthetic gasoline was used to mimic the real gasoline sample matrix. Gasoline is highly hydrophobic, flammable and volatile, therefore, a sample pretreatment that will make it less hydrophobic and volatile prior to the preconcentration step, is required. In this work detergentless microemulsion procedure was used. The procedure for the preparation of gasoline–ethanol–water mixture was carried out according to Ozcan and coworkers.34 The mixture was spiked with 1.0 mL of a 1.0 mg L−1 multi-element standard solution and made up to the mark with ethanol to obtain 10 μg L−1 concentration of each metal ion. The mixture was homogenized by shaking using vortex shaker.The sample solution (20 mL) was pumped through the μ-SPE column at an appropriate flow rate (5–10 mL min−1) by means of a peristaltic pump. The flow rates less than five were not investigated to avoid long analysis time. After sample loading, the μ-SPE device was washed with ultrapure water to remove excess organic matrix, followed by 1.0 mL of ammonium acetate buffer solution to remove major cations (Na, Ca, K, etc.), at 7.5 mL min−1.15,16 The elution of the retained metal ions was archived by pumping an appropriate volume (0.5–2.0 mL) of nitric acid (1.0–3.0 mol L−1), at a flow rates of 0.5–1.5 mL min−1 and was aspirated directly to the nebulizer of the ICP-MS instrument. The above procedure was applied for the analysis of blank solutions and real samples. In between the experiments, the column was washed with 2 mL ultrapure water followed by conditioning with 2 mL ammonium acetate buffer (1.0 M, pH 9.0) and then with 2 mL ethanol at 7.5 mL min−1.
2.7 Optimization approach
There are several factors that affect the separation and preconcentration of metal ions in different samples matrices. These factors include mass of the sorbent (MS), sample pH, preconcentration flow rate (PFR) eluent concentration (EC), eluent volume (EV) and eluent flow rate (EFR). With the intention of obtaining the optimum conditions of μ-SPE for preconcentration of metal ions in gasoline samples, a two level fractional factorial design (26–2) was used for the screening of the significant factors. The latter were further optimized by Box–Behnken design. Maximum, central point and minimum levels in Table 2 for each variable were chosen according to data from preliminary investigation. All the experiments were carried out in triplicates. The experiments were performed in random order and the experimental data was processed using STATISTICA software.| Variable | Low level (−1) | Central point (0) | High level (+1) |
|---|---|---|---|
| MS (mg) | 30 | 65 | 100 |
| pH | 5 | 7.5 | 10 |
| EC(mol L−1) | 1 | 2 | 3 |
| EV (mL) | 0.5 | 1.25 | 2 |
| PFR (mL min−1) | 5 | 7.5 | 10 |
| EFR (mL min−1) | 0.5 | 1 | 1.5 |
3 Results and discussion
3.1 Characterization and functionalization of the nanometer-sized alumina
The surface and textural morphology (SEM image) of nano-Al2O3 obtained by the sol–gel method starting from aluminum chloride as a precursor is illustrated in Fig. 1. The SEM image showed fine particles and their diameter was estimated to be about 2 nm up to a maximum of 60 nm.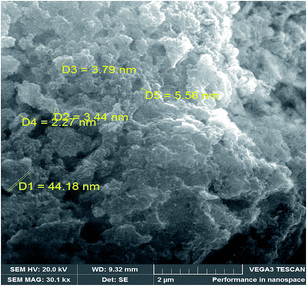 | ||
| Fig. 1 Scanning electron microscopy images of alumina obtained by sol–gel methods starting from AlCl3 as precursor, calcined at 1000 °C for three hours. | ||
The BET results revealed that the nano-Al2O3 adsorbent has porous characteristics with a remarkable specific surface area of 312 m2 g−1. The relative high specific surface area revealed the availability of adsorbent sites for metal ions preconcentration (adsorption). This phenomenon is significant for the application of Al2O3 in the separation and preconcentration system for the determination of metals in an organic matrix. Furthermore, the relatively high specific surface area represents substantial developments over ion-exchange adsorbents such as Chelex-100.
X-ray diffraction pattern (for 2θ diffraction angles from 10° to 80°) of the nano-Al2O3 calcined at 1000 °C for 3 hours is presented in Fig. 2. The XRD pattern showed the crystalline structure of the nanometer-sized particles indicating various peaks indexed to alumina.35,36 The peaks were attributed to two crystallization phases of alumina, that is, α-Al2O3 and γ-Al2O3.
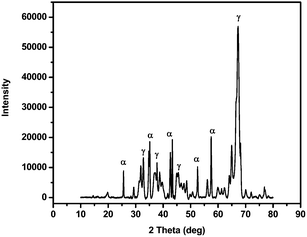 | ||
| Fig. 2 X-ray diffraction pattern of alumina obtained by sol–gel methods starting from AlCl3 as precursor, calcined at 1000 °C for three hour. | ||
The functionalized nano-Al2O3 was characterization with ATR-FTIR spectroscopy. The infrared spectrum of nano-Al2O3/AAPTMS showed a characteristic bands at 3576 and 2924 cm−1 which was attributed to stretching vibrations of N–H and C–H groups, respectively. In the addition, bending vibration of N–H group, was observed around 1570 cm−1. These observations confirm that nano-Al2O3 has been successfully functionalized with AAPTMS.
3.2 Multivariate optimization using factorial design
Optimization of the μ-SPE method was carried out in two consecutive steps, that is, a two-level fractional factorial (26−2) and Box–Behnken designs. The fraction factorial design (FFD) was used for screening of the significant factors. In the FFD, six variables, mass of the sorbent (MS), sample pH, preconcentration flow rate (PFR) eluent concentration (EC), eluent volume (EV) and eluent flow rate (EFR), were selected based on the preliminary studies. The 26−2 experimental design for the studied factors and the analytical responses are shown in Table S1.† The effects of the investigated variables were studied using analysis of variance (ANOVA) and the data are presented in the form of Pareto charts (Fig. S1–S5†). The screening results showed that for simultaneous preconcentration, MS, EV and EFR were the most statistically significant factors for almost all the investigated analytes. The effect of sample pH, EC and PFR on the analytical response was not considerable except for Cr where sample pH was statistically significant. Therefore, for simultaneous preconcentration, sample pH, eluent concentration and preconcentration flow rate were maintained at 7.5, 2.0 mol L−1 and 7.5 mL min−1, respectively.The overall results obtained for the screening analysis using 26−2 fractional factorial experimental design indicated that mass of the sorbent, eluent volume and eluent flow rate require a final optimization. In order to provide maximum recovery of the studied analytes, Box–Behnken design containing a total of 15 experiments (Table S2†) was carried out to optimize these three variables. The 3D surface responses (Fig S6–S10†) of the quadratic models were used to evaluate the interactive relationships between independent variables (mass of the sorbent and eluent volume) and the response. Based on quadratic equations resulted from the 3D surface response plots, the calculation indicated that 85 mg, 1.5 mL and 0.7 mL min−1 for MS, EV and EFR, respectively, provided maximum retention and recovery of the all the studied analytes.
3.3 Adsorption capacities of metal ions
The adsorption capacity is one of the important factors, as it determines the amount of sorbent required to quantitatively preconconcentrate the analytes from a given solution.37 Preliminary adsorption studies with nano-Al2O3/AAPTMS revealed that 15 minutes is adequate time for the system to reach equilibrium. Therefore, 0.1 g nano-Al2O3/AAPTMS was equilibrated in 50 mL of Co, Cr, Mn, Ni and Ti ethanol solutions at concentrations 30 to 250 mg L−1 by shaking for 35 minutes at pH 7.5. The amount of metal ions in solution was determined by GFAAS. The experimental data were fitted into the general equation of the modified Langmuir model presented in eqn (1).38 The later was used to calculate the adsorption capacities for each metal ion.
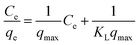 | (1) |
The results showed that adsorption capacity of the analytes probably differ due to their size, degree of hydration and the value of their binding constant with nano-Al2O3/AAPTMS.39,40 The maximum sorption capacities were found to be 15.8, 23.8, 32.3, 31.1 and 35.7 mg g−1 for Co, Cr, Mn, Ni and Ti, respectively.
3.4 Regeneration of the adsorbent
The stability and regeneration possibility of the nano-Al2O3/AAPTMS adsorbent were investigated (Table S3†). The adsorbent can be reused after regeneration with 1.0 mL HNO3 (2.0 mol L−1) solution and 1.0 mL ultrapure water, respectively. In addition the nano-Al2O3/AAPTMS adsorbent was relatively stable up to 45 runs without noticeable decrease in the recoveries for the all the target analytes.3.5 Analytical performance of the μ-SPE-ICP-MS method
The analytical performances of the developed μ-SPE-ICP-MS method under optimum conditions for preconcentration and determination of metal ion were evaluated and the results are presented in Table 3. The limit of detection (LOD) and the limit of quantification (LOQ) were defined as LOD=3Sd/m and LOQ=10Sd/m, respectively, where m and Sd are the slope of the analytical curve and the standard deviation of 20 consecutive measurements of the blank signal, respectively. For 20 mL of sample solution used, LOD and LOQ (in the original sample) for Co, Cr, Mn, Ni, and Ti are presented in Table 3. The overall precision (repeatability) of the SPE method, expressed as relative standard deviation (n = 15, 10 μg L−1), was found to be ≤2%. The reproducibility of the proposed μ-SPE-ICP-MS method was evaluated over a period of ten working days. In each day the five determinations of a model sample containing 20 μg L−1 target analytes were performed. It should be noted that the μ-SPE-ICP-MS system was freshly prepared for each day. The obtained RSD was about 4.5%.| Analytes | Sensitivity (cps L μg−1) | Correlation coefficient | LOD (ng L−1) | LOQ (ng L−1) | Precision (%RSD) |
|---|---|---|---|---|---|
| Co | 41.6 | 0.9991 | 11.5 | 38.3 | 1.9 |
| Cr | 58.7 | 0.9968 | 9.8 | 32.6 | 1.7 |
| Mn | 94.5 | 0.9973 | 6.7 | 22.3 | 1.1 |
| Ni | 121.1 | 0.9982 | 4.4 | 14.7 | 1.1 |
| Ti | 153.2 | 0.9961 | 2.3 | 7.7 | 1.3 |
Larger sample volumes containing trace amounts of the analytes were used in order to get the highest enrichment factor. Therefore, various volumes (20–150 mL) of the model sample containing 10 μg L−1 of each metal were analyzed using the optimized method. The quantitative recoveries (≥95%) were obtained for sample volumes up 100 mL for the studied metal ions; under these conditions the preconcentration factor of 20 was achieved. However, to process 100 mL of a sample would take about 15 min which then translate to 4 samples per hour. Therefore, a sample volume of 20 mL was used and the preconcentration factor was 4.
The overall time required for the preconcentration of 20 mL sample ((2.7 min, at a flow rate of 7.5 mL min−1), cleaning (approximately 0.3 min at a flow rate of 7.5 mL min−1), elution (2.1 min at a rate of 0.7 mL min−1), and conditioning (0.3 min at a flow rate of 7.5 mL min−1)) was about 5.4 min. Therefore the sampling frequency (sample throughput) was about 11 samples per hour.
A comparison of the analytical performance data of the developed μ-SPE-ICP-MS method with other approaches reported based on the preconcentration systems coupled to ICP-MS and other methods are given in Table 4. The analytical figures of merit obtained revealed that the proposed μ-SPE-ICP-MS method has relatively low LOD when compared with those reported in the literature (Table 4). However, the LODs for Co and Ni were higher than those reported by Su et al.41 Oliveira et al.42 and Hu et al.23 The adsorption capacities on the other hand were better than those reported by Yin et al.17
| Method | Sample | Analyte | LOD ng L−1 | Adsorbents | PF | AC | Reference |
|---|---|---|---|---|---|---|---|
| a SPE-ICP-MS = solid phase extraction coupled to inductively coupled plasma mass spectrometry, USN = ultrasonic nebulizer, ETV = electrothermal vaporization, μSPE = micro-solid-phase extraction, PFW = petroleum produced formation water. | |||||||
| SPE-ICP-MS | Rice and water | Co, Cr, Mn, Ni | 38 (Co), 15 (Cr), 6.7 (Mn), 45 (Ni) | Nanometer-sized alumina | 5 | 10 (Co), 14 (Cr), 16 (Mn), 13 (Ni) | Yin et al.17 |
| USN-ICP-MS | Gasoline | Co, Mn, Ni, Ti | 100 (Co), 600 (Mn), 100 (Ni), 700 (Ti) | — | — | — | Duyck et al.3 |
| ETV-ICP-MS | Gasoline | Mn, Ni | 20 (Mn), 380 (Ni) | — | — | — | Saint'Pierre et al.1 |
| SPE-ICP-MS | Urine and seawater | Co | 0.7 | PVC beads | — | — | Su et al.41 |
| SPE-ICP-MS | PFW | Co, Mn | 2 (Co), 17 (Mn) | Toyopearl AF-Chelate-650M iminodiacetate resin | — | — | Oliveira et al.42 |
| SPE/ICP-MS | Water | Cr, Ti | Cr (40), Ti (110) | 2-Nitroso-1-naphthol-MCI GEL CHP20P resin | 20 | — | Aydin and Soylak43 |
| SPE-ICP-MS | Water, rice and urine | Co, Ni | 0.3 (Co), 1.5 (Ni) | Mesoporous Al2O3 | 10 | — | Hu et al.23 |
| μSPE-ICP-MS | Gasoline | Co, Cr, Mn, Ni, Ti | 11.5 (Co), 9.8 (Cr), 6.7 (Mn), 4.4 (Ni), 2.3 (Ti) | Nano-Al2O3/AAPTMS | 4 | 16 (Co), 24 (Cr), 32 (Mn), 31 (Ni), 36 (Ti) | This work |
3.6 Interference studies
Under optimized conditions, the effect of the potential interfering ions on the extraction efficiency of the target elements was evaluated. Model solutions containing 20 μg L−1 the analytes of interest and 200 μg L−1 of other metal cations normally found in fuel samples, were prepared and analyzed by the proposed μ-SPE-ICP-MS method. The metallic cations included Ag(I), Cd(II), Cu(II), Fe(II), Pb(II) and Zn(II). These metallic cations were chosen due to the fact that the can also be retained by the adsorbent. The analytical results (Table S4†) obtained demonstrated that the above mentioned interfering ions did not interference with the quantification of even at concentration higher than those normally found in gasoline.3.7 Validation of the μ-SPE-ICP-MS method
Due to the absence of certified reference material (CRM) that is similar to the investigated samples, the accuracy of μ-SPE-ICP-MS method was examined by standard addition method. Gasoline sample (1-MFUG) was spiked with organic and inorganic standard solutions. In addition, the aim of spiking the gasoline sample with organic and inorganic standard solutions was to evaluate the nano-Al2O3/AAPTMS sorption efficiency to different metal species in gasoline. This because trace element forms in petroleum products is not fully known and different species may display different adsorption behaviors.10,15 As it can be seen in Table 5, similar percentage recoveries were obtained for organic and inorganic forms. This implied that μ-SPE-ICP-MS system may be used for the sorption of trace elements in their inorganic or metal-organic forms.15 In addition, the results obtained (Table 5), confirmed the accuracy of the preconcentration method, taking into consideration that the recoveries were in the range from 97–101%.| Addedc | Co | Cr | Mn | Ni | Ti | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Foundc | %Rd | Found | %R | Found | %R | Found | %R | Found | %R | ||
| a IS: Inorganic standard.b MOS = metallo-organic standard.c Concentration in μg L−1; Recovery in %.d Average ± standard deviation. | |||||||||||
| ISa | 0 | 6.8 ± 0.7d | — | 77.3 ± 1.6 | — | 84.9 ± 0.9 | — | 38.3 ± 1.1 | — | 4.1 ± 0.6 | — |
| 5 | 11.6 ± 1.4 | 96.0 ± 1.8 | 82.1 ± 2.2 | 97.2 ± 1.6 | 90.0 ± 1.1 | 102 ± 1.5 | 43.2 ± 1.2 | 98.5 ± 2.1 | 8.9 ± 1.7 | 96.6 ± 1.5 | |
| 20 | 26.3 ± 1.5 | 97.5 ± 2.5 | 97.0 ± 1.2 | 98.4 ± 1.2 | 104.8 ± 2.1 | 99.5 ± 1.7 | 58.2 ± 1.4 | 99.5 ± 1.8 | 23.8 ± 1.3 | 98.5 ± 0.7 | |
| MOSb | 0 | 6.9 ± 1.2 | — | 77.1 ± 1.7 | — | 85.0 ± 0.9 | — | 38.6 ± 1.2 | — | 3.9 ± 0.4 | — |
| 5 | 11.8 ± 0.9 | 98.0 ± 2.3 | 81.9 ± 1.0 | 95.6 ± 3.4 | 89.9 ± 1.2 | 98.0 ± 2.1 | 43.5 ± 1.0 | 98.7 ± 1.6 | 8.8 ± 1.1 | 97.2 ± 0.8 | |
| 20 | 26.5 ± 1.4 | 98.0 ± 1.8 | 96.8 ± 1.0 | 98.4 ± 1.4 | 104.7 ± 1.3 | 98.7 ± 2.0 | 58.4 ± 0.97 | 99.1 ± 0.9 | 23.7 ± 1.5 | 99.0 ± 1.2 | |
3.8 Application of μ-SPE-ICP-MS method
The μ-SPE-ICP-MS method was applied for the quantification of Co, Cr, Mn, Ni, and Ti in commercial gasoline samples purchased from six different filling stations in Johannesburg, as presented in Table 6. As shown in this table, the concentrations of Co are quite low (<20 μg L−1) for almost all the samples except for 2-MCUG sample (26.7 μg L−1). The concentration of Cr was found to be relatively high (<50 μg L−1) in 1-MFUG, 2-MCUG and 3-MCUG samples. The Mn concentrations were generally high in all the samples. Thus, the highest concentration was 38.2 mg L−1 and the lowest was 85.8 μg L−1. The concentration of Ni ranged from 6.74 to 195.4 μg L−1 and Ti content was relatively low except for 2-MCUG and 6-MCUG samples. In addition, it can be seen from Table 6 that Co, Cr and Ti could not be quantified in some of the samples, as their concentrations were found to be below the LOD. It is worthwhile mentioning that Mn in samples 2-MCUG, 3-MCUG and 6-MCUG was used as a fuel additive, and it was therefore present in both free and organic compound forms (methylcyclopentadienyl manganese tricarbonyl, MMT). It should be noted that for determination of Mn in 2-MCUG, 3-MCUG and 6-MCUG samples, the latter were further diluted to obtain μg L−1 range.| Sample ID | Co | Cr | Mn | Ni | Ti |
|---|---|---|---|---|---|
| a MFUG: metal-free unleaded gasoline.b MCUG: metal-containing unleaded gasoline.c ND: not detectable.d Concentration in mg L−1; 1–6 are the numbers allocated to the six gasoline filling stations.e Average ± standard deviation. | |||||
| 1-MFUGa | 6.8 ± 0.7e | 77.3 ± 1.6 | 84.9 ± 0.9 | 38.3 ± 1.1 | 4.1 ± 0.6 |
| 2-MCUGb | 26.7 ± 0.8 | 59.4 ± 0.1 | 18.3 ± 1.2d | 75.3 ± 0.6 | 25.8 ± 1.2 |
| 2-MFUG | 6.9 ± 0.3 | 13.3 ± 0.8 | 95.7 ± 1.0 | 20.7 ± 0.3 | ND |
| 3-MCUG | 6.1 ± 0.5 | 71.2 ± 1.9 | 38.7 ± 2.6d | 67.8 ± 0.7 | 15.3 ± 0.7 |
| 3-MFUG | NDc | ND | 152 ± 1.3 | 16.5 ± 0.8 | ND |
| 4-MFUG | 3.8 ± 0.2 | 4.0 ± 0.1 | 94.3 ± 0.6 | 23.8 ± 1.1 | ND |
| 5-MCUG | 16.5 ± 0.3 | ND | 106 ± 1.4 | 194.6 ± 2.1 | ND |
| 5-MFUG | ND | ND | 111 ± 2.0 | 7.1 ± 0. 6 | ND |
| 6-MCUG | ND | 31.9 ± 0.3 | 21.0 ± 2.0d | 55.7 ± 0.9 | 41.3 ± 1.4 |
| 6-MFUG | ND | 21.3 ± 0.2 | 120.8 ± 3.4 | 31.4 ± 1.0 | 13.5 ± 0.3 |
4 Conclusions
A simple and effective method for the preconcentration determination of metal ions in gasoline samples by μ-SPE-ICP-MS using nano-Al2O3/AAPTMS as a sorbent material was developed. The coupling of a μ-SPE system with ICP-MS reduced the factors that are generally encountered in batch procedures; these include longer analysis time, high sample consumption and contamination risks. In addition, the preconcentration step eliminated matrix interferences encountered in the analysis of organic matrices using ICP-MS and resulted in the improved sensitivity. Furthermore, the use of multivariate optimization technique reduced the number of experimental run, thus reducing the total analysis time. The developed μ-SPE-ICP-MS method was found to be simple, cost effective, efficient, precise and accurate since results obtained with the analysis of spiked samples presented good agreement with the added values.Acknowledgements
The authors wish to thank Sasol and National Research Foundation for financial assistance. University of Johannesburg (Spectrum) is acknowledged for providing ICP-MS facilities.References
- T. D. Saint'Pierre, L. F. Dias, D. Pozebon, R. Q. Aucélio, A. J. Curtius and B. Welz, Spectrochim. Acta, Part B, 2002, 57, 1991–2001 CrossRef.
- G. Caumette, C.-P. Lienemann, I. Merdrignac, H. Paucot, B. Bouyssiere and R. Lobinski, Talanta, 2009, 80, 1039–1043 CrossRef CAS PubMed.
- C. Duyck, N. Miekeley, C. L. Porto da Silveira and P. Szatmari, Spectrochim. Acta, Part B, 2002, 57, 1979–1990 CrossRef.
- C. P. Lienemann, S. Dreyfus, C. Pecheyran and O. F. X. Donard, Oil Gas Sci. Technol. – Rev. IFP, 2007, 62, 69–77 CrossRef CAS.
- E. Bjorn and W. Frech, Anal. Bioanal. Chem., 2003, 376, 274–278 Search PubMed.
- E. S. Chaves, F. G. Lepri, J. S. A. Silva, D. P. C. de Quadros, T. D. Saint'Pierre and A. J. Curtius, J. Environ. Monit., 2008, 10, 1211–1216 RSC.
- T. D. Saint'pierre, L. F. Dias, S. M. Maia and A. J. Curtius, Spectrochim. Acta, Part B, 2004, 59, 551–558 CrossRef.
- P. S. Roldan, I. L. Alcântara, J. C. Rocha, C. C. F. Padilha and P. M. Padilha, Ecl. Quím. São Paulo, 2004, 29, 33–40 CrossRef CAS.
- D. S. S. Santos, M. G. A. Korn, M. A. B. Guida, G. L. dos Santos, V. A. Lemos and L. S. G. Teixeira, J. Braz. Chem. Soc., 2011, 22, 552–557 CrossRef CAS.
- L. S. G. Teixeira, M. d. A. Bezerra, V. A. Lemos, H. C. d. Santos, D. S. de Jesus and A. C. S. Costa, Sep. Sci. Technol., 2005, 40, 555–2565 CrossRef.
- L. S. G. Teixeira, R. B. S. Rocha, E. V. Sobrinho, P. R. B. Guimarães, L. A. M. Pontes and J. S. R. Teixeira, Talanta, 2007, 72, 1073–1076 CrossRef CAS PubMed.
- P. S. Roldan, I. L. Alcântara, G. R. Castro, J. C. Rocha, C. C. F. Padilha and P. M. Padilha, Anal. Bioanal. Chem., 2003, 375, 574 CAS.
- V. N. Alves, R. Mosquetta, N. M. M. Coelho, J. N. Bianchin, K. C. Di Pietro Roux, E. Martendal and E. Carasek, Talanta, 2010, 80, 1133–1138 CrossRef CAS PubMed.
- P. S. Roldan, I. L. Alcântara, C. C. F. Padilha and P. M. Padilha, Fuel, 2005, 84, 305–309 CrossRef CAS.
- P. N. Nomngongo, J. Catherine Ngila, J. N. Kamau, T. A. M. Msagati, L. Marjanovic and B. Moodley, Anal. Chim. Acta, 2013, 787, 78–86 CrossRef CAS PubMed.
- P. N. Nomngongo, J. C. Ngila, J. N. Kamau, T. A. M. Msagati and B. Moodley, Talanta, 2013, 110, 153–159 CrossRef CAS PubMed.
- J. Yin, Z. Jiang, G. Chang and B. Hu, Anal. Chim. Acta, 2005, 540, 333–339 CrossRef CAS.
- O. Abollino, M. Aceto, C. Sarzanini and E. Mentasti, Anal. Chim. Acta, 2000, 411, 223–237 CrossRef CAS.
- W. S. Khayoon, B. Saad, B. Salleh, N. H. A. Manaf and A. A. Latiff, Food Chem., 2014, 147, 287–294 CrossRef CAS PubMed.
- J. Meng, J. Bu, C. Deng and X. Zhang, J. Chromatogr. A, 2011, 1218, 1585–1591 CrossRef CAS PubMed.
- C. Basheer, A. A. Alnedhary, B. S. M. Rao, S. Valliyaveettil and H. K. Lee, Anal. Chem., 2006, 78, 2853–2858 CrossRef CAS PubMed.
- C. Basheer, H. G. Chong, T. M. Hii and H. K. Lee, Anal. Chem., 2007, 79, 6845–6850 CrossRef CAS PubMed.
- W. Hu, B. Hu and Z. Jiang, Anal. Chim. Acta, 2006, 572, 55–62 CrossRef CAS PubMed.
- F.-Y. Zheng, S.-X. Li, L.-X. Lin and L.-Q. Cheng, J. Hazard. Mater., 2009, 172, 618–622 CrossRef CAS PubMed.
- Y. Liu, P. Liang and L. Guo, Talanta, 2005, 68, 25–30 CrossRef CAS PubMed.
- M. Hua, S. Zhang, B. Pan, W. Zhang, L. Lv and Q. Zhang, J. Hazard. Mater., 2012, 211–212, 317–331 CrossRef CAS PubMed.
- G. Chang, Z. Jiang, T. Peng and B. Hu, Acta Chim. Sin., 2003, 61, 100–103 CAS.
- A. M. Haji Shabani, S. Dadfarnia and Z. Dehghani, Talanta, 2009, 79, 1066–1070 CrossRef PubMed.
- M. Ghaedi, K. Niknam, A. Shokrollahi, E. Niknam, H. R. Rajabi and M. Soylak, J. Hazard. Mater., 2008, 155, 121–127 CrossRef CAS PubMed.
- A. Afkhami, M. Saber-Tehrani and H. Bagheri, J. Hazard. Mater., 2010, 181, 836–844 CrossRef CAS PubMed.
- R. Rogojan, E. Andronescu, C. Ghitulica and B. S. Vasile, U. P. B. Sci. Bull. Series B, 2011, 73, 65–76 Search PubMed.
- C. Hang, B. Hu, Z. Jiang and N. Zhang, Talanta, 2007, 71, 1239–1245 CrossRef CAS PubMed.
- C. R. T. Tarley, G. F. Lima, D. R. Nascimento, A. R. S. Assis, E. S. Ribeiro, K. M. Diniz, M. A. Bezerra and M. G. Segatelli, Talanta, 2012, 100, 71–79 CrossRef CAS PubMed.
- M. Ozcan and S. Akman, Spectrochim. Acta, Part B, 2005, 60, 399–402 CrossRef.
- C. H. Shek, J. K. L. Lai, T. S. Gu and G. M. Lin, Nanostruct. Mater., 1997, 8, 605–610 CrossRef CAS.
- T. A. Saleh and V. K. Gupta, Sep. Purif. Technol., 2012, 89, 245–251 CrossRef CAS.
- Ş. Tokalıoğlu, V. Yılmaz and Ş. Kartal, Environ. Monit. Assess., 2009, 152, 369–377 CrossRef PubMed.
- R. Qu, C. Sun, F. Ma, Z. Cui, Y. Zhang, X. Sun, C. Ji, C. Wang and P. Yin, Fuel, 2012, 92, 204–210 CrossRef CAS.
- M. Shishehbore, A. Afkhami and H. Bagheri, Chem. Cent. J., 2011, 5, 1–10 CrossRef PubMed.
- P. N. Nomngongo, J. C. Ngila, S. M. Musyoka, T. A. M. Msagati and B. Moodley, Anal. Methods, 2013, 5, 3000–3008 RSC.
- C.-K. Su, T.-W. Lee and Y.-C. Sun, J. Anal. At. Spectrom., 2012, 27, 1585–1590 RSC.
- E. P. Oliveira, L. Yang, R. E. Sturgeon, R. E. Santelli, M. A. Bezerra, S. N. Willie and R. Capilla, J. Anal. At. Spectrom., 2011, 26, 578–585 RSC.
- F. A. Aydin and M. Soylak, J. Hazard. Mater., 2010, 173, 669–674 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra07074g |
| This journal is © The Royal Society of Chemistry 2014 |
