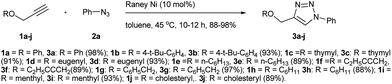
Raney Ni catalyzed azide-alkyne cycloaddition reaction†
H. Surya Prakash Rao* and
Guravaiah Chakibanda
Department of Chemistry, Pondicherry University, Puducherry-605 014, India. E-mail: hspr.che@pondiuni.edu.in
First published on 10th September 2014
Abstract
Raney Ni efficiently catalyzes acetylene azide cycloaddition reactions to form 1,2,3-triazoles. Unlike the CuSO4/sodium ascorbate reagent system, there is no need for a reducing agent under Raney Ni catalysis. Terminal acetylene selectivity, 1,4-regioselectivity and mild reaction conditions are prominent features of the method. Mechanistic probing revealed that the reaction does not go through nickel acetylides.
1. Introduction
The Huisgen's 1,3-dipolar cycloaddition of azides to alkynes for the synthesis of 1,2,3-triazoles is one of the most prominent name reactions.1 The reaction has become most applied for covalently linking two divergent molecular entities: one with a terminal acetylene group and the other with an azide,2 owing to independent discoveries by Sharpless and Meldal that copper(I)3 catalysts efficiently promote azide alkyne cycloaddition (AAC) in high yield as well as in a regio-(1,4- over 1,5-) and chemo-(terminal alkyne vs. internal alkyne reactivity) selective manner. The copper(I) mediated azide acetylene cycloaddition (CuAAC) reactions have become premier examples for a click reaction as they provide near quantitative yield of the regiochemically pure 1,4-disubstituted 1,2,3-triazoles, tolerate a wide variety of functional groups and can be conducted under mild reaction conditions including in aqueous medium. The CuAAC reaction found applications4 in diversified fields such as medicinal,5 polymer,6 materials7 and bioorganic8 etc. Instead of employing Cu(I) complexes or its salts, which are relatively unstable or difficult to prepare, the CuAAC reactions are generally conducted with a catalytic amount of CuSO4 and sodium ascorbate where sodium ascorbate serves as the reducing agent for in situ generation of Cu(I) species. According to well-accepted mechanism Cu(I) species gets intimately involved in every stage of the reaction and helps to bring azide and acetylene units in close proximity for cycloaddition to take place. Although the click reactions with CuSO4 and sodium ascorbate have become hugely popular, still, the need for a reducing agent is one of the disadvantages of the method. Alternatively, some catalysts derived from Ag,9 Au,10 Al,11 Ru,12 and Ir13 complexes which work without additional reducing agent have been employed to promote AAC, but the catalysts are difficult to make or expensive and most of the times reactions are cumbersome to conduct. Thus, there is a need to discover an alternative catalyst, which is inexpensive, readily available, reusable and does not require an additional reducing agent.2. Results and discussion
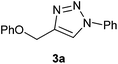
In a quest to discover such a catalyst, we screened several bench top stable and inexpensive salts like Ni(OAc)2, NiCl2, NiCO3, NiSO4, BiCl3, BiNO3, As2O3 and Sb2O3 in catalytic amounts (10 mol%) for cycloaddition of phenyl propargyl ether 1a to phenyl azide 2a to provide triazole 3a (Scheme 1). None of these catalysts worked independent of a reducing agent. The nickel salts like Ni(OAc)2, NiCl2, NiCO3 and NiSO4, catalysed the AAC reaction and provided triazole 3a in moderate yield (<70%) in presence of 20 mol% of the reducing agents like hydrazine, glucose, lactose or particularly sodium ascorbate. Outcome of the reaction indicated that reactive Ni(0) species could be the catalyst in the reaction. Therefore, in sequel, we employed Raney Ni as the catalyst and discovered that it catalyses the AAC efficiently (Scheme 1). Notably, there was no need for a reducing agent like sodium ascorbate when Raney Ni was employed. Raney Ni is a classical reagent used extensively as a catalyst for hydrogenation of carbon–carbon and carbon-hetero atom double or triple bonds.14 It is also used as a reagent for desulfurization. Thus, in continuation of our studies on the Huisgen reaction,15 herein we report scope and limitations as well as some evidence for mechanism of the Raney Ni mediated AAC for a facile synthesis of several 1,2,3-traizoles.The cycloaddition of phenyl propargyl ether 1a to phenyl azide 2a to give cycloadduct 3a under 10 mol% of Raney Ni catalysis was selected to optimize the reaction conditions (Scheme 1, Fig. 1). Of different solvents like H2O (15 h, 70%), t-BuOH/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2; 15 h, 82%), MeOH (16 h, 90%), DMF (16 h, 90%), dioxane (16 h, 87%) and toluene (10 h, 98%) tried, the reaction worked best in toluene. Stirring the heterogeneous reaction mixture at 45 °C for 12 h led to complete reaction in 98% yield as evidenced by the absence of phenyl propargyl ether 1a in TLC. A study to determine optimal temperature for the reaction revealed that 45 °C is ideal for high yield and exclusive regioselectivity (1,4-substitution over 1,5-substitution) of the product. Although at higher temperatures still, both the rate of the reaction and yield of the triazole were higher (99%), the regio-chemical purity of the product was lower. For example, at 100 °C the reaction took 1 h for completion but the product has 2
2; 15 h, 82%), MeOH (16 h, 90%), DMF (16 h, 90%), dioxane (16 h, 87%) and toluene (10 h, 98%) tried, the reaction worked best in toluene. Stirring the heterogeneous reaction mixture at 45 °C for 12 h led to complete reaction in 98% yield as evidenced by the absence of phenyl propargyl ether 1a in TLC. A study to determine optimal temperature for the reaction revealed that 45 °C is ideal for high yield and exclusive regioselectivity (1,4-substitution over 1,5-substitution) of the product. Although at higher temperatures still, both the rate of the reaction and yield of the triazole were higher (99%), the regio-chemical purity of the product was lower. For example, at 100 °C the reaction took 1 h for completion but the product has 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 1,4- and 1,5-regioisomers. At room temperature (30 °C) the reaction took longer time for completion (24 h, 80%). The fact that Raney Ni is practically immiscible with toluene was used for the recovery of the product and to recycle the catalyst. After each reaction, the reaction mixture was centrifuged in a laboratory centrifuge at 2100 rpm for 1 min. Supernatant solution was separated and the settled Raney Ni cake was reused. The catalytic activity remained excellent for two runs (94–98%). On further use, the yield started to decrease (84% in the third run) with a concurrent increase in the time required to complete the reaction. Concentration of copper impurities in the Raney Ni sample was analyzed using ICP-MS to evaluate if such copper impurities are responsible for AAC reaction. Using ICP-MS analysis technique one can identify copper, for that matter, most of the metal impurities present even below ppb levels.16 The analysis clearly showed that the Raney Ni sample we were employing does not have copper impurities up to ppb level. This result made us conclude that Ni is responsible for the AAC reaction.
1 mixture of 1,4- and 1,5-regioisomers. At room temperature (30 °C) the reaction took longer time for completion (24 h, 80%). The fact that Raney Ni is practically immiscible with toluene was used for the recovery of the product and to recycle the catalyst. After each reaction, the reaction mixture was centrifuged in a laboratory centrifuge at 2100 rpm for 1 min. Supernatant solution was separated and the settled Raney Ni cake was reused. The catalytic activity remained excellent for two runs (94–98%). On further use, the yield started to decrease (84% in the third run) with a concurrent increase in the time required to complete the reaction. Concentration of copper impurities in the Raney Ni sample was analyzed using ICP-MS to evaluate if such copper impurities are responsible for AAC reaction. Using ICP-MS analysis technique one can identify copper, for that matter, most of the metal impurities present even below ppb levels.16 The analysis clearly showed that the Raney Ni sample we were employing does not have copper impurities up to ppb level. This result made us conclude that Ni is responsible for the AAC reaction.
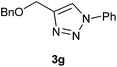
Building on the results from development of optimal conditions for the NiAAC reaction of phenyl propargyl ether 1a to phenyl azide 2a to give the regiochemically selective triazole 3a, we wanted to probe the effect of different substitution on propargyl ethers and azides. Nine propargyl ethers, namely, 4-t-butylphenyl propargyl ether 1b, thymol propargyl ether 1c, eugenol propargyl ether 1d, n-hexyl propargyl ether 1e, 1-(prop-2-ynyloxy)pent-2-yl propargyl ether 1f, benzyl propargyl ether 1g, cyclohexyl propargyl ether 1h, menthyl propargyl ether 1i, cholesteryl propargyl ether 1j were reacted with phenyl azide 2a to realize exclusive formation of regiochemically pure triazoles 3b–j, in excellent yield. The propargyl ethers were selected for their structural diversity of being derived from an alcohol group present on aromatic, benzylic, aliphatic or natural product scaffolds. Significantly, the NiAAC was not affected in terms of yield or regioselectivity by subtle changes in steric and electronic environment in the aryl ring of the propargyl ethers. Among all the products listed in Scheme 1, 3f is interesting as it proved that similar to CuAAC reactions, the NiAAC takes place on terminal alkyne rather than internal alkyne.
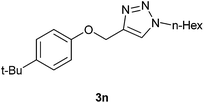
Next, it was our endeavour to evaluate the efficiency of Raney Ni catalyst in the regioselectivity in the cycloaddition of benzyl and hexyl azides to aryl propargyl ethers. Accordingly two aryl propargyl ethers, namely phenyl propargyl ether 1a and 4-t-butylphenyl propargyl ether 1b were subjected to Raney Ni catalyzed AAC with benzyl azide 2b, and hexyl azide 2c in combinatorial fashion to realize four 1,4-disubstituted triazoles 3k–n in near quantitative yield with exclusive regiochemistry (Scheme 2).
 | ||
| Scheme 2 Raney Ni mediated combinatorial cycloaddition of two aryl propargyl ethers to benzyl and n-hexyl azides. | ||
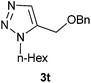
The NiAAC reaction of benzyl 1c and cyclohexyl 1d propargyl ethers to benzyl 2b and n-hexyl 2c azides was conducted in combinatorial fashion to evaluate efficiency of the catalyst for promoting the reaction of alkyl propargyl ethers and alkyl azides (Table 1). Raney Ni aided the cycloaddition of benzyl 2b and n-hexyl 2c azides to propargylic ethers 1c–d efficiently in providing cycloadducts 3o–v in excellent yield. But, the regiochemistry, unfortunately, was scrambled to some extent with the formation of major 1,4-regio isomers 3o–r and along with the corresponding minor 1,5-regioisomers 3s–v (Table 1). Ratios of the regio-isomers were calculated on the basis of integration of relevant signals in the mixture 1H NMR and 13C NMR spectra. The results indicate that two transition states that lead to 1,4- or 1,5-disubstituted traizoles may not have much difference in free energy and thus both orientations as shown in proposed mechanism are possible (Scheme 4) (Table 2).
 | ||
| Scheme 3 Accepted mechanism for the CuAAC.21 | ||
Finally, we conducted NiAAC of phenyl azide 2a with three alkynes namely (((1-ethynylcyclohexyl)oxy)methyl)benzene 1e, phenyl acetylene 1f and propargyl alcohol 1g to evaluate versatility of NiAAC reaction. The NiAAC reaction of propagyl ether 1e, where C(1) carbon is disubstituted, provided the cycloadduct 4-(1-(benzyloxy)cyclohexyl)-1-phenyl-1H-1,2,3-triazole 3w exclusively, indicating that the reaction is not effected by steric hindrance close to the reaction site. The NiAAC reaction of phenyl acetylene 1f with phenyl azide provided regiochemically pure 1,4-diphenyl-1H-1,2,3-triazole17 3x indicating that NiAAC reaction can be extended to aryl acetylenes. The NiAAC reaction of propargyl alcohol 1g and phenyl azide, however, provided the 1,4- and 1,5-regioisomeric triazolyl methanols 3y and 3z in the ratio of about 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The AAC reaction of propargyl alcohol 1g and phenyl azide 2a without any catalyst also provided the 1,4- and 1,5-regioisomeric adducts 3y–z in 3
2. The AAC reaction of propargyl alcohol 1g and phenyl azide 2a without any catalyst also provided the 1,4- and 1,5-regioisomeric adducts 3y–z in 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio but the reaction required heating by microwaves to 140 °C in polyethylene glycol-200 for 2 min. The CuAAC reaction of propargyl alcohol with phenyl azide, on the other hand, provided 1,4-regioisomer 3y exclusively.18 The results indicate that AAC under Ni catalysis takes place at much lower temperature (45 °C vs. 140 °C) and since NiAAC reaction provided both the regioisomers it could go through a mechanistic pathway different from CuAAC.
2 ratio but the reaction required heating by microwaves to 140 °C in polyethylene glycol-200 for 2 min. The CuAAC reaction of propargyl alcohol with phenyl azide, on the other hand, provided 1,4-regioisomer 3y exclusively.18 The results indicate that AAC under Ni catalysis takes place at much lower temperature (45 °C vs. 140 °C) and since NiAAC reaction provided both the regioisomers it could go through a mechanistic pathway different from CuAAC.
One of the principal and initial intermediates in the Sharpless–Meldal version of the CuAAC reaction is the copper acetylide 5 generated by oxidative insertion of Cu(I) into CH bond of a terminal alkyne 4 (Scheme 3). This step appears to be crucial to the cycloaddition of azides to the acetylenic triple bond. In the next step copper gets involved in bringing together copper acetylide and the azide through π-complexation with alkyne and coordination to the terminal nitrogen of the azide. The cycloaddition leads to organocopper intermediate 6, which gets hydrolysed to provide 3 on addition of water. However, in confined places like in zeolites, there may not be copper acetylide formation and the cycloaddition could go through metallocycle.19 The Ni(0) catalyzed AAC reaction could also work in a similar fashion, initiated by Ni acetylide formation. Or, Ni(0) could play a role in bringing the reactants together though π-complexation and coordination. To probe the mechanism of the NiAAC reaction, experiments with deuterated phenyl propargyl ether ((((3-deuteroprop-2-yn-1-yl)oxy)methyl)benzene)20 4 with phenyl azide 2a were performed. The deuterium present on terminal acetylenic carbon gets lost, if the reaction goes through Ni acetylide formation. On the other hand, if the Ni(0) plays the role of a coordinating species deuterium is retained. In the Ni(0) catalyzed AAC reaction, we observed almost 100% retention of deuterium in the product 8 (Scheme 4). This result indicates that unlike that of CuAAC, in the first step, Ni(0) does not insert into CH of alkyne to form Ni acetylide. Mechanistically, the reaction could go through complexation of the acetylene on Raney Ni surface to form π-complex 7 (Scheme 4) followed by cycloaddition of the azides to form adduct 9 via complex 8 where both acetylene and azide components are held by Ni. On the other hand, the Ni complex with acetylene moiety could undergo cycloaddition with azide moiety in the orientation as shown in 10. Steric and electronic characteristics of the azide and to some extent those of propargyl ether make the transition states 8 or 10 energetically more favourable to provide 1,4-adduct in preference to 1,5-adduct.
3. Conclusion
In conclusion, we have demonstrated that Raney Ni is an alternative catalyst to copper(II) sulphate and sodium ascorbate recipe for effecting AAC reaction. Notably, additional reducing agent is not required when Raney Ni is used. Moreover, the recovery of the products by the present method is greatly facilitated, because simple centrifugation and solvent evaporation provide pure 1,4-disubstituted 1,2,3-triazoles. In most of the cases, exclusive 1,4-disubstitution in triazole products is observed. Mechanistic probing indicated that Raney Ni plays the role of coordinating species in bringing together azide and alkyne.4. Experimental section
4.1. General
Analytical thin-layer chromatography (TLC) was performed on silica gel coated on glass plates (0.25 mm, silica gel G, LOBA Chemicals, UV silica gel GF 254). TLC spots were visualized under UV light and iodine. Column chromatography was carried out using silica gel 100–200 mesh (LOBA Chemicals) using a hexanes–ethyl acetate eluent mixture. 1H and 13C NMR spectra were recorded on a Bruker Avance 400 spectrometer in CDCl3 + CCl4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and chemical shifts values (δ) are relative to the residual solvent peak (δ 7.26 for 1H, δ 77.16 for 13C) where possible or alternatively to TMS (δ 0.00) as internal standard. Coupling constants (J) are given in Hz and multiplicities are designated as s (singlet), d (doublet), dd (doublet of doublet), t (triplet), q (quartet), m (multiplet). NMR spectra were recorded for all the samples to determine the number of hydrogen atoms present on each carbon. Although spectral data was obtained for all the compounds prepared in the present study, spectral data of only unknown compounds are provided here. Melting points were determined using open-ended capillary tubes on VEEGO VMP-DS instrument and are uncorrected. The propargyl ethers were prepared according to the literature procedure from the corresponding alcohol or phenols.22 Phenyl, benzyl and hexyl azides23 were prepared by following the literature procedures. 3a,24 3k,25 3m,26 3l,27 3x,17 3y,18 3c, 3d, 3i and 3j15 are known compounds. Spectral data of the unknown compounds excepting 3a is given here. Where mixture of regioisomers were obtained, spectral data of the 1,4-regioisomer culled from the mixture NMR spectral data was given. Approximately 10 mol% of Raney Ni present as a suspension in dry EtOH was transferred into dry nitrogen flushed RB flask. Excess EtOH was removed under reduced pressure using Schlenk line. The amount of reactants was calculated on the basis of the accurate weight of Raney Ni taken in the RB flask. The reactants were dissolved in dry toluene before transferring into the RB. Contents of the RB with constant stirring were heated to 45 °C in a preheated oil-bath. We evaluated if any Raney Ni catalyst leached by taking the NiAAC of phenyl acetylene and phenyl azide as standard and found that less than 0.4% of the catalyst reduced weight in each of the three consecutive runs (see ESI for details†). Although we cannot rule out absence of leaching, we attribute decrease in weight of Raney Ni to experimental error.
1) and chemical shifts values (δ) are relative to the residual solvent peak (δ 7.26 for 1H, δ 77.16 for 13C) where possible or alternatively to TMS (δ 0.00) as internal standard. Coupling constants (J) are given in Hz and multiplicities are designated as s (singlet), d (doublet), dd (doublet of doublet), t (triplet), q (quartet), m (multiplet). NMR spectra were recorded for all the samples to determine the number of hydrogen atoms present on each carbon. Although spectral data was obtained for all the compounds prepared in the present study, spectral data of only unknown compounds are provided here. Melting points were determined using open-ended capillary tubes on VEEGO VMP-DS instrument and are uncorrected. The propargyl ethers were prepared according to the literature procedure from the corresponding alcohol or phenols.22 Phenyl, benzyl and hexyl azides23 were prepared by following the literature procedures. 3a,24 3k,25 3m,26 3l,27 3x,17 3y,18 3c, 3d, 3i and 3j15 are known compounds. Spectral data of the unknown compounds excepting 3a is given here. Where mixture of regioisomers were obtained, spectral data of the 1,4-regioisomer culled from the mixture NMR spectral data was given. Approximately 10 mol% of Raney Ni present as a suspension in dry EtOH was transferred into dry nitrogen flushed RB flask. Excess EtOH was removed under reduced pressure using Schlenk line. The amount of reactants was calculated on the basis of the accurate weight of Raney Ni taken in the RB flask. The reactants were dissolved in dry toluene before transferring into the RB. Contents of the RB with constant stirring were heated to 45 °C in a preheated oil-bath. We evaluated if any Raney Ni catalyst leached by taking the NiAAC of phenyl acetylene and phenyl azide as standard and found that less than 0.4% of the catalyst reduced weight in each of the three consecutive runs (see ESI for details†). Although we cannot rule out absence of leaching, we attribute decrease in weight of Raney Ni to experimental error.
4.2. ICP-MS analysis of Raney Ni for detection of trace amounts of copper
Five samples each having 10 mL of 460 ppb Raney Ni digested in aq 2 N HNO3 (milli-Q water of specific resistance less than 15 mΩ) and spiked with 0, 1, 2, 3, 4 mL of 24.4 ppb Cu containing CuSO4·5H2O solution, were prepared and analyzed in ICP-MS (Thermo Scientific, X SERIES 2) for determination of Cu content at ppb levels in blank by calibration method28 (Fig. 1). The data points which fit into linear scale (three) were taken into consideration while drawing Fig. 1.The analysis did not indicate presence of even ppt amounts of copper in the Raney Ni sample used in the NiAAC reactions.
4.3. Preparation of Raney Ni
Approximately 10 mol% of Raney Ni present as a suspension in absolute EtOH was transferred into dry nitrogen flushed RB flask. Excess EtOH was removed under reduced pressure using Schlenk line. The amount of reactants was calculated on the basis of the accurate weight of Raney Ni taken in the RB flask. To Raney Ni (42 mg, 0.7 mmol) the azide (7.0 mmol) and then the propargyl ether (7.0 mmol) dissolved in dry toluene (10 mL each) were added. The reaction mixture was placed in a preheated oil bath maintained at 45 °C and stirred for 14 h by which time reaction was complete (TLC). Contents of the flask was transferred into centrifuge tubes and centrifuged for 2 min at 2100 rpm. Supernatant clear solution was withdrawn followed by washing of the catalyst twice with 10 mL dry toluene. Removal of toluene from pooled solutions resulted in triazoles 3 in over 90% yield which was sufficiently pure for spectral characterization. Column chromatography was performed when necessary.4.4. General procedure for the Raney Ni catalyzed [3 + 2] cycloaddition of azides and terminal alkynes
To Raney Ni (42 mg, 0.7 mmol) taken under a blanket of dry nitrogen, phenyl azide (1.0 g, 7.0 mmol) and then phenyl propargyl ether (1.0 g, 7.0 mmol) dissolved in dry toluene (10 mL each) were added. The reaction mixture was placed in a preheated oil bath maintained at 45 °C. The reaction mixture was stirred for 14 h by which time reaction was complete (TLC). Contents of the flask was transferred into a centrifuge tube and centrifuged for 2 min at 2100 rpm. Supernatant clear solution was withdrawn followed by washing of the catalyst twice with 10 mL dry toluene. Removal of toluene from pooled solutions resulted is 3a in 98% yield (186 mg) as a colorless viscous liquid, which was sufficiently pure for spectral and analytical characterization. IR (KBr) 3112, 3059, 3032, 1741, 1455, 1357, 1246, 1080, 752 cm−1; 1H NMR (CDCl3 + CCl4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 400 MHz) δ 8.05 (s, 1H), 7.75–7.72 (m, 2H), 7.55–7.44 (m, 3H), 7.33–7.29 (m, 2H), 7.04–6.97 (m, 3H), 5.31 (s, 2H) ppm; 13C NMR (CDCl3 + CCl4 (1
1), 400 MHz) δ 8.05 (s, 1H), 7.75–7.72 (m, 2H), 7.55–7.44 (m, 3H), 7.33–7.29 (m, 2H), 7.04–6.97 (m, 3H), 5.31 (s, 2H) ppm; 13C NMR (CDCl3 + CCl4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 100 MHz) δ 158.3, 129.9, 129.7, 129.0, 121.5, 120.7, 114.9, 62.1 ppm.
1), 100 MHz) δ 158.3, 129.9, 129.7, 129.0, 121.5, 120.7, 114.9, 62.1 ppm.
Yield 93% (151 mg), white solid, mp 68 °C; IR (KBr): 3156, 3043, 2966, 2869, 1606, 1512, 1244, 1048, 858, 711, 548 cm−1; 1H NMR (CDCl3 + CCl4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 400 MHz) δ 8.02 (s, 1H), 7.76–7.74 (m, 2H), 7.54–7.49 (m, 2H), 7.44–7.40 (m, 1H), 7.30–7.28 (m, 2H), 6.93–6.91 (m, 2H), 5.28 (s, 2H), 1.31 (s, 9H) ppm; 13C NMR (CDCl3 + CCl4 (1
1), 400 MHz) δ 8.02 (s, 1H), 7.76–7.74 (m, 2H), 7.54–7.49 (m, 2H), 7.44–7.40 (m, 1H), 7.30–7.28 (m, 2H), 6.93–6.91 (m, 2H), 5.28 (s, 2H), 1.31 (s, 9H) ppm; 13C NMR (CDCl3 + CCl4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 100 MHz) δ 155.8, 145.3, 143.7, 137.0, 129.5, 128.5, 126.2, 120.4, 120.2, 114.1, 62.0, 34.0, 31.5 ppm; HRMS m/z (ESI-MS): calcd for C19H21N3ONa (M + Na) 330.1582, found 330.1580.
1), 100 MHz) δ 155.8, 145.3, 143.7, 137.0, 129.5, 128.5, 126.2, 120.4, 120.2, 114.1, 62.0, 34.0, 31.5 ppm; HRMS m/z (ESI-MS): calcd for C19H21N3ONa (M + Na) 330.1582, found 330.1580.
Yield 89% (164 mg),viscous liquid, IR (KBr) 3079, 3016, 2924, 2853, 1593, 1509, 1491, 1453, 693 cm−1; 1H NMR (CDCl3 + CCl4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 400 MHz) δ 7.75 (s, 1H), 7.66–7.63 (m, 2H), 7.53–7.50 (m, 3H), 4.48 (s, 2H), 3.44 (t, J = 4.0 Hz, 2H), 1.58–1.54 (m, 2H), 1.31–1.27 (m, 6H), 0.88 (t, J = 4.0 Hz, 3H) ppm; 13C NMR (CDCl3 + CCl4 (1
1), 400 MHz) δ 7.75 (s, 1H), 7.66–7.63 (m, 2H), 7.53–7.50 (m, 3H), 4.48 (s, 2H), 3.44 (t, J = 4.0 Hz, 2H), 1.58–1.54 (m, 2H), 1.31–1.27 (m, 6H), 0.88 (t, J = 4.0 Hz, 3H) ppm; 13C NMR (CDCl3 + CCl4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 100 MHz) δ 136.6, 135.0, 133.9, 129.5, 124.7, 70.9, 60.8, 31.8, 29.7, 26.0, 22.8, 14.2 ppm; HRMS m/z (ESI-MS): (M + H) calcd for C15H22N3O 260.1763, found 260.1763.
1), 100 MHz) δ 136.6, 135.0, 133.9, 129.5, 124.7, 70.9, 60.8, 31.8, 29.7, 26.0, 22.8, 14.2 ppm; HRMS m/z (ESI-MS): (M + H) calcd for C15H22N3O 260.1763, found 260.1763.
Yield 89% (175 mg), viscous liquid, IR (KBr) 3081, 3052, 3016, 2921, 2851, 1598, 1509, 1493, 1451, 1432, 696 cm−1; 1H NMR (CDCl3 + CCl4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 400 MHz) δ 7.98 (s, 1H), 7.72 (d, J = 8.3 Hz, 2H), 7.50 (t, J = 8.0 Hz, 2H), 7.41 (t, J = 6.9 Hz, 1H), 4.78 (s, 2H), 4.23 (s, 2H), 2.25–2.23 (m, 2H), 1.15 (t, J = 8.0 Hz, 3H) ppm; 13C NMR (CDCl3 + CCl4 (1
1), 400 MHz) δ 7.98 (s, 1H), 7.72 (d, J = 8.3 Hz, 2H), 7.50 (t, J = 8.0 Hz, 2H), 7.41 (t, J = 6.9 Hz, 1H), 4.78 (s, 2H), 4.23 (s, 2H), 2.25–2.23 (m, 2H), 1.15 (t, J = 8.0 Hz, 3H) ppm; 13C NMR (CDCl3 + CCl4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 100 MHz) δ 145.7, 137.2, 129.8, 128.8, 120.8, 120.6, 89.1, 74.9, 63.0, 58.4, 13.9, 12.6 ppm; HRMS m/z (ESI-MS): calcd for C14H15N3ONa (M + Na) 264.1113, found 264.1113.
1), 100 MHz) δ 145.7, 137.2, 129.8, 128.8, 120.8, 120.6, 89.1, 74.9, 63.0, 58.4, 13.9, 12.6 ppm; HRMS m/z (ESI-MS): calcd for C14H15N3ONa (M + Na) 264.1113, found 264.1113.
Yield 97% (176 mg), viscous liquid, IR (KBr) 3103, 2956, 2923, 2863, 1504, 1285, 1098, 1061, 768, 692, 587 cm−1; 1H NMR (CDCl3 + CCl4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 400 MHz) δ 7.96 (s, 1H), 7.74–7.71 (m, 2H), 7.52–7.43 (m, 2H), 7.43–7.27 (m, 6H), 4.75 (s, 2H), 4.64 (s, 2H) ppm; 13C NMR (CDCl3 + CCl4 (1
1), 400 MHz) δ 7.96 (s, 1H), 7.74–7.71 (m, 2H), 7.52–7.43 (m, 2H), 7.43–7.27 (m, 6H), 4.75 (s, 2H), 4.64 (s, 2H) ppm; 13C NMR (CDCl3 + CCl4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 100 MHz) δ 146.0, 137.7, 137.1, 129.6, 128.5, 128.4, 127.8, 127.7, 120.4, 120.3, 72.6, 63.6 ppm; HRMS m/z (ESI-MS): 288.1113 calcd for C16H15N3ONa (M + Na) 288.1112.
1), 100 MHz) δ 146.0, 137.7, 137.1, 129.6, 128.5, 128.4, 127.8, 127.7, 120.4, 120.3, 72.6, 63.6 ppm; HRMS m/z (ESI-MS): 288.1113 calcd for C16H15N3ONa (M + Na) 288.1112.
Yield 88% (163 mg), viscous liquid, IR (KBr) 3032, 2932, 2856, 1605, 1496, 1453, 1363, 1082, 722 cm−1; 1H NMR (CDCl3 + CCl4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 400 MHz) δ 7.80 (s, 1H), 7.66–7.64 (m, 2H), 7.55–7.50 (m, 3H), 4.53 (s, 2H), 3.34–3.29 (m, 1H), 1.84–1.69 (m, 6H), 1.53–1.51 (m, 1H), 1.31–1.20 (m, 3H) ppm; 13C NMR (CDCl3 + CCl4 (1
1), 400 MHz) δ 7.80 (s, 1H), 7.66–7.64 (m, 2H), 7.55–7.50 (m, 3H), 4.53 (s, 2H), 3.34–3.29 (m, 1H), 1.84–1.69 (m, 6H), 1.53–1.51 (m, 1H), 1.31–1.20 (m, 3H) ppm; 13C NMR (CDCl3 + CCl4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 100 MHz) δ 147.2, 137.4, 129.8, 128.7, 120.6, 120.3, 77.6, 61.7, 32.4, 26.0, 24.2 ppm; HRMS m/z (ESI-MS): calcd for C15H20N3O (M + H) 258.1606, found 258.1602.
1), 100 MHz) δ 147.2, 137.4, 129.8, 128.7, 120.6, 120.3, 77.6, 61.7, 32.4, 26.0, 24.2 ppm; HRMS m/z (ESI-MS): calcd for C15H20N3O (M + H) 258.1606, found 258.1602.
Yield 93% (155 mg), white solid, mp 69 °C; IR (KBr): 3155, 2963, 1604, 1510, 1463, 1243, 1045, 822, 710, 547 cm−1; 1H NMR (CDCl3 + CCl4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 400 MHz) δ 7.52 (s, 1H), 7.23 (dd, J = 8.4 Hz, 2H), 6.85 (dd, J = 8.6, 1.4 Hz, 2H), 5.12 (d, J = 2.5 Hz, 2H), 4.28–4.24 (m, 2H), 1.84 (t, J = 5.6 Hz, 2H), 1.26 (s, 9H), 1.25 (s, 6H), 0.84 (t, J = 1.6 Hz, 3H) ppm; 13C NMR (CDCl3 + CCl4 (1
1), 400 MHz) δ 7.52 (s, 1H), 7.23 (dd, J = 8.4 Hz, 2H), 6.85 (dd, J = 8.6, 1.4 Hz, 2H), 5.12 (d, J = 2.5 Hz, 2H), 4.28–4.24 (m, 2H), 1.84 (t, J = 5.6 Hz, 2H), 1.26 (s, 9H), 1.25 (s, 6H), 0.84 (t, J = 1.6 Hz, 3H) ppm; 13C NMR (CDCl3 + CCl4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 100 MHz) δ 156.0, 144.2, 143.6, 126.2, 122.3, 114.2, 62.1, 50.2, 34.1, 31.2, 31.1, 30.2, 26.1, 22.4, 13.9 ppm; HRMS m/z (ESI-MS): calcd For C19H29N3ONa (M + Na) 338.2208, found 338.2196.
1), 100 MHz) δ 156.0, 144.2, 143.6, 126.2, 122.3, 114.2, 62.1, 50.2, 34.1, 31.2, 31.1, 30.2, 26.1, 22.4, 13.9 ppm; HRMS m/z (ESI-MS): calcd For C19H29N3ONa (M + Na) 338.2208, found 338.2196.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) 3o, 3s.
1) 3o, 3s. The regioisomers 3o and 3s were obtained in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.7 ratio. Overall yield 93% (177 mg), viscous liquid, IR (KBr) 3139, 3066, 2930, 2860, 1597, 1494, 1240, 755 cm−1; NMR spectral data of the 1,5-regio isomer was culled from the mixture NMR spectra by locating the signals meant for 1,4-regioisomer.29
0.7 ratio. Overall yield 93% (177 mg), viscous liquid, IR (KBr) 3139, 3066, 2930, 2860, 1597, 1494, 1240, 755 cm−1; NMR spectral data of the 1,5-regio isomer was culled from the mixture NMR spectra by locating the signals meant for 1,4-regioisomer.29
4.4.9.1. 1-Benzyl-5-(benzyloxymethyl)-1H-1,2,3-triazole 3s.
Yield 89%, viscous liquid, IR (KBr) 3139, 3066, 2930, 2860, 1597, 1494, 1240, 755 cm−1; 1H NMR (CDCl3 + CCl4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 400 MHz) 7.32 (s, 1H), 7.28–7.07 (m, 10H), 5.42 (s, 2H), 4.56 (s, 2H), 4.30 (s, 2H), 13C NMR 137.9, 133.0, 134.8, 133.0, 129.0, 128.7, 28.50, 128.35, 128.30, 128.1, 127.7, 72.4, 59.8, 52.4 ppm; HRMS m/z (ESI-MS): calcd for C17H18N3O (M + H) 280.1450, found 280.1452.
1), 400 MHz) 7.32 (s, 1H), 7.28–7.07 (m, 10H), 5.42 (s, 2H), 4.56 (s, 2H), 4.30 (s, 2H), 13C NMR 137.9, 133.0, 134.8, 133.0, 129.0, 128.7, 28.50, 128.35, 128.30, 128.1, 127.7, 72.4, 59.8, 52.4 ppm; HRMS m/z (ESI-MS): calcd for C17H18N3O (M + H) 280.1450, found 280.1452.
The regioisomers 3p and 3t were obtained in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 ratio. Overall yield 93% (173 mg), viscous liquid, IR (KBr) 3032, 3061, 2958, 2926, 2856, 1597, 1489, 1243, 755 cm−1; NMR spectral data of the 1,5-regio isomer was identified from the mixture NMR by deducting the spectrum meant for 1,4-regio-isomer. The 1,4-regioisomer 3p was prepared by Cu(I) mediated cycloaddition of benzyl propargyl ether and n-hexylazide by following the general procedure described by us previously.15
0.2 ratio. Overall yield 93% (173 mg), viscous liquid, IR (KBr) 3032, 3061, 2958, 2926, 2856, 1597, 1489, 1243, 755 cm−1; NMR spectral data of the 1,5-regio isomer was identified from the mixture NMR by deducting the spectrum meant for 1,4-regio-isomer. The 1,4-regioisomer 3p was prepared by Cu(I) mediated cycloaddition of benzyl propargyl ether and n-hexylazide by following the general procedure described by us previously.15
4.4.10.1. 4-(Benzyloxymethyl)-1-hexyl-1H-1,2,3-triazole 3p.
Yield 98%, viscous liquid, IR (KBr) 3137, 3065, 2926, 2858, 1649, 1496, 1240, 736 cm−1; 1H NMR (CDCl3 + CCl4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 400 MHz) 7.48 (s, 1H), 7.28–7.25 (m, 5H), 4.60 (s, 2H), 4.52 (s, 2H), 4.26–4.22 (t, J = 7.2 Hz, 2H), 1.82–1.79 (m, 2H), 1.27–1.22 (m, 6H), 0.85–0.82 (m, 3H) ppm; 13C NMR 144.8, 137.7, 128.3, 128.1, 122.1, 72.1, 63.4, 49.9, 31.0, 30.9, 25.9, 22.2, 13.7 ppm.
1), 400 MHz) 7.48 (s, 1H), 7.28–7.25 (m, 5H), 4.60 (s, 2H), 4.52 (s, 2H), 4.26–4.22 (t, J = 7.2 Hz, 2H), 1.82–1.79 (m, 2H), 1.27–1.22 (m, 6H), 0.85–0.82 (m, 3H) ppm; 13C NMR 144.8, 137.7, 128.3, 128.1, 122.1, 72.1, 63.4, 49.9, 31.0, 30.9, 25.9, 22.2, 13.7 ppm.
4.4.10.2. 5-(Benzyloxymethyl)-1-hexyl-1H-1,2,3-triazole 3t.
Yield 89%, viscous liquid, IR (KBr) 3081, 3052, 3016, 2921, 2851, 1598, 1509, 1493, 1451, 1432, 696 cm−1; 1H NMR (CDCl3 + CCl4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 400 MHz) δ 7.48 (s, 1H), 7.27–7.25 (m, 5H), 4.60 (d, J = 0.4 Hz, 2H), 4.52 (s, 2H), 1.82–1.79 (m, 2H), 1.25–1.22 (m, 7H) ppm; 13C NMR (CDCl3 + CCl4 (1
1), 400 MHz) δ 7.48 (s, 1H), 7.27–7.25 (m, 5H), 4.60 (d, J = 0.4 Hz, 2H), 4.52 (s, 2H), 1.82–1.79 (m, 2H), 1.25–1.22 (m, 7H) ppm; 13C NMR (CDCl3 + CCl4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 100 MHz) δ 144.7, 137.7, 128.3, 127.8, 127.7, 122.1, 72.1, 63.4, 49.9, 30.99, 30.93, 30.02, 25.9, 22.2, 13.7 ppm; HRMS m/z (ESI-MS): calcd for C16H23N3ONa (M + Na) 296.1738, found 296.1723.
1), 100 MHz) δ 144.7, 137.7, 128.3, 127.8, 127.7, 122.1, 72.1, 63.4, 49.9, 30.99, 30.93, 30.02, 25.9, 22.2, 13.7 ppm; HRMS m/z (ESI-MS): calcd for C16H23N3ONa (M + Na) 296.1738, found 296.1723.
The regioisomers 3q and 3u were obtained in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. Overall yield 91% (178 mg), viscous liquid, IR (KBr) 3031, 2931, 2852, 1497, 1450, 1356, 1082 cm−1; NMR spectral data of the 1,5-regio isomer was identified from the mixture NMR by deducting the spectrum meant for 1,4-regio-isomer. The 1,4-regioisomer 3q was prepared by Cu(I) mediated cycloaddition of benzyl propargyl ether and n-hexylazide by following the general procedure described by us previously.15
1 ratio. Overall yield 91% (178 mg), viscous liquid, IR (KBr) 3031, 2931, 2852, 1497, 1450, 1356, 1082 cm−1; NMR spectral data of the 1,5-regio isomer was identified from the mixture NMR by deducting the spectrum meant for 1,4-regio-isomer. The 1,4-regioisomer 3q was prepared by Cu(I) mediated cycloaddition of benzyl propargyl ether and n-hexylazide by following the general procedure described by us previously.15
4.4.11.1. 1-Benzyl-4-(cyclohexyloxymethyl)-1H-1,2,3-triazole 3q.
Yield 97%, white solid, mp 91–93 °C, IR (KBr) 3140, 2930, 2857, 1454, 1368, 1089, 953, 730 cm−1; 1H NMR (CDCl3 + CCl4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 400 MHz) 7.40 (s, 1H), 7.33–7.32 (m, 3H), 7.24–7.23 (m, 2H) 5.47 (s, 2H), 4.60 (s, 2H), 3.35–3.32 (m, 1H), 1.90–1.87 (m, 2H), 1.71–1.69 (m, 2H), 1.51–1.50 (m, 1H), 1.28–1.19 (m, 2H) ppm; 13C NMR (CDCl3 + CCl4 (1
1), 400 MHz) 7.40 (s, 1H), 7.33–7.32 (m, 3H), 7.24–7.23 (m, 2H) 5.47 (s, 2H), 4.60 (s, 2H), 3.35–3.32 (m, 1H), 1.90–1.87 (m, 2H), 1.71–1.69 (m, 2H), 1.51–1.50 (m, 1H), 1.28–1.19 (m, 2H) ppm; 13C NMR (CDCl3 + CCl4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 100 MHz) 146.7, 134.8, 129.2, 128.8, 128.2, 122.1, 61.7, 54.2, 32.3, 25.9, 24.2 ppm.
1), 100 MHz) 146.7, 134.8, 129.2, 128.8, 128.2, 122.1, 61.7, 54.2, 32.3, 25.9, 24.2 ppm.
4.4.11.2. 1-Benzyl-5-(cyclohexyloxymethyl)-1H-1,2,3-triazole 3u.
Yield 89%, viscous liquid, IR (KBr) 3031, 2931, 2852, 1497, 1450, 1356, 1082 cm−1; 1H NMR (CDCl3 + CCl4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 400 MHz) δ 7.55 (s, 1H), 7.34–7.28 (m, 5H), 5.59 (s, 2H), 4.60 (s, 2H), 3.21–3.17 (m, 1H), 1.87–1.69 (m, 5H), 1.27–1.20 (m, 5H) ppm; 13C NMR (CDCl3 + CCl4 (1
1), 400 MHz) δ 7.55 (s, 1H), 7.34–7.28 (m, 5H), 5.59 (s, 2H), 4.60 (s, 2H), 3.21–3.17 (m, 1H), 1.87–1.69 (m, 5H), 1.27–1.20 (m, 5H) ppm; 13C NMR (CDCl3 + CCl4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 100 MHz) δ 146.8, 134.8, 134.0, 133.9, 128.2, 127.5, 77.4, 76.8, 57.9, 52.2, 31.9, 25.7, 23.9 ppm; HRMS m/z (ESI-MS): calcd for C16H21N3ONa (M + Na) 294.1582, found 294.1584.
1), 100 MHz) δ 146.8, 134.8, 134.0, 133.9, 128.2, 127.5, 77.4, 76.8, 57.9, 52.2, 31.9, 25.7, 23.9 ppm; HRMS m/z (ESI-MS): calcd for C16H21N3ONa (M + Na) 294.1582, found 294.1584.
The regioisomers 3r and 3v were obtained in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 ratio. Overall yield 89% (170 mg), viscous liquid, IR (KBr) 3034, 2987, 2854, 1605, 1496, 1453, 1082, 722 cm−1; NMR spectral data of the 1,5-regio isomer was identified from the mixture NMR by deducting the spectrum meant for 1,4-regio-isomer. The 1,4-regioisomer 3r was prepared by Cu(I) mediated cycloaddition of benzyl propargyl ether and n-hexylazide by following the general procedure described by us previously.15
0.5 ratio. Overall yield 89% (170 mg), viscous liquid, IR (KBr) 3034, 2987, 2854, 1605, 1496, 1453, 1082, 722 cm−1; NMR spectral data of the 1,5-regio isomer was identified from the mixture NMR by deducting the spectrum meant for 1,4-regio-isomer. The 1,4-regioisomer 3r was prepared by Cu(I) mediated cycloaddition of benzyl propargyl ether and n-hexylazide by following the general procedure described by us previously.15
4.4.12.1. 4-(Cyclohexyloxymethyl)-1-hexyl-1H-1,2,3-triazole 3r.
Yield 98%, viscous liquid, IR (KBr) 3137, 3065, 2926, 2858, 1496, 1458, 1368, 1072, 736 cm−1; 1H NMR (CDCl3 + CCl4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 400 MHz) 7.42 (s, 1H), 4.52 (s, 2H), 4.22 (t, J = 7.2 Hz, 2H), 3.29 (m, 1H), 1.79 (d, J = 9.2 Hz, 5H), 1.61 (s, 3H), 1.43–1.41 (m, 2H), 1.20–1.15 (m, 5H), 0.77 (s, 3H) ppm; 13C NMR (CDCl3 + CCl4 (1
1), 400 MHz) 7.42 (s, 1H), 4.52 (s, 2H), 4.22 (t, J = 7.2 Hz, 2H), 3.29 (m, 1H), 1.79 (d, J = 9.2 Hz, 5H), 1.61 (s, 3H), 1.43–1.41 (m, 2H), 1.20–1.15 (m, 5H), 0.77 (s, 3H) ppm; 13C NMR (CDCl3 + CCl4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 100 MHz) 146.0, 121.9, 77.4, 76.8, 69.8, 61.4, 50.1, 35.4, 31.1, 26.0, 23.9, 22.3, 13.8 ppm.
1), 100 MHz) 146.0, 121.9, 77.4, 76.8, 69.8, 61.4, 50.1, 35.4, 31.1, 26.0, 23.9, 22.3, 13.8 ppm.
4.4.12.2. 5-(Cyclohexyloxymethyl)-1-hexyl-1H-1,2,3-triazole 3v.
Yield 91%, viscous liquid, IR (KBr) 3034, 2987, 2854, 1605, 1496, 1453, 1082, 722 cm−1; 1H NMR (CDCl3 + CCl4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 400 MHz) δ 7.57 (s, 1H), 4.66 (s, 2H), 4.35–4.30 (m, 3H), 3.41–3.31 (m, 1H), 1.74–1.72 (m, 5H), 1.31–1.21 (m, 12H), 0.88–0.85 (m, 3H) ppm; 13C NMR (CDCl3 + CCl4 (1
1), 400 MHz) δ 7.57 (s, 1H), 4.66 (s, 2H), 4.35–4.30 (m, 3H), 3.41–3.31 (m, 1H), 1.74–1.72 (m, 5H), 1.31–1.21 (m, 12H), 0.88–0.85 (m, 3H) ppm; 13C NMR (CDCl3 + CCl4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 100 MHz) δ 146.3, 122.1, 61.7, 50.4, 48.6, 32.3, 31.4, 30.3, 26.4, 25.8, 25.7, 24.2, 23.9, 22.5, 14.0 ppm; HRMS m/z (ESI-MS): calcd for C15H27N3ONa (M + Na) 288.2052, found 288.2050.
1), 100 MHz) δ 146.3, 122.1, 61.7, 50.4, 48.6, 32.3, 31.4, 30.3, 26.4, 25.8, 25.7, 24.2, 23.9, 22.5, 14.0 ppm; HRMS m/z (ESI-MS): calcd for C15H27N3ONa (M + Na) 288.2052, found 288.2050.
Yield 89% (133 mg), white solid, mp 93 °C; IR (KBr): 3029, 2933, 2862, 1493, 1450, 719 cm−1; 1H NMR (CDCl3 + CCl4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 400 MHz) δ 7.91 (s, 1H), 7.70 (d, 2H, 1.2 Hz), 7.67–7.42 (m, 2H), 7.40–7.33 (m, 2H), 7.31–7.7.27 (m, 3H), 7.24–7.16 (m, 1H), 4.32 (s, 2H), 2.24–2.06 (m, 2H), 1.80–1.76 (m, 5H), 1.59–1.52 (m, 1H) ppm; 13C NMR (CDCl3 + CCl4 (1
1), 400 MHz) δ 7.91 (s, 1H), 7.70 (d, 2H, 1.2 Hz), 7.67–7.42 (m, 2H), 7.40–7.33 (m, 2H), 7.31–7.7.27 (m, 3H), 7.24–7.16 (m, 1H), 4.32 (s, 2H), 2.24–2.06 (m, 2H), 1.80–1.76 (m, 5H), 1.59–1.52 (m, 1H) ppm; 13C NMR (CDCl3 + CCl4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 100 MHz) δ 152.7, 139.2, 137, 129.5, 128.3, 128.0, 127.0, 126.9, 120.2, 119.0, 73.9, 35.1, 25.5, 21.9 ppm. HRMS m/z (ESI-MS): calcd For C21H23N3O Na (M + Na) 356.1733, found 356.1735.
1), 100 MHz) δ 152.7, 139.2, 137, 129.5, 128.3, 128.0, 127.0, 126.9, 120.2, 119.0, 73.9, 35.1, 25.5, 21.9 ppm. HRMS m/z (ESI-MS): calcd For C21H23N3O Na (M + Na) 356.1733, found 356.1735.
Yield 93% (135 mg); The NiAAC reaction of phenyl azide and propargyl alcohol provided 1,4-3y and 1,5-3z regioisomers in 64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36 ratio. The AAC reaction without any catalyst provided 1,4-3y and 1,5-3z regioisomers in the same ratio but the reaction required heating in a monomode microwave oven to 140 °C for 2 min in polyethylene glycol 200 (PEG 200) medium. Authentic 1,4-disubstituted triazole (DT) was prepared by heating a mixture of phenyl azide and propargyl alcohol under microwave irradiation at 140 °C for 2 min in PEG-200 medium. The NMR spectral assignments for 1,4-3y and 1,5-3z DTs were based on the 13C NMR spectral analysis as described by Dondoni and coworkers.30 Signals belonging to the 1,4-regioisomer (major product) and the 1,5-regioisomer31 elicited from the 1H and 13C NMR spectra of mixture and presented here.1 1,4-Regioisomer 3y: 1H NMR (CDCl3 + CCl4 (1
36 ratio. The AAC reaction without any catalyst provided 1,4-3y and 1,5-3z regioisomers in the same ratio but the reaction required heating in a monomode microwave oven to 140 °C for 2 min in polyethylene glycol 200 (PEG 200) medium. Authentic 1,4-disubstituted triazole (DT) was prepared by heating a mixture of phenyl azide and propargyl alcohol under microwave irradiation at 140 °C for 2 min in PEG-200 medium. The NMR spectral assignments for 1,4-3y and 1,5-3z DTs were based on the 13C NMR spectral analysis as described by Dondoni and coworkers.30 Signals belonging to the 1,4-regioisomer (major product) and the 1,5-regioisomer31 elicited from the 1H and 13C NMR spectra of mixture and presented here.1 1,4-Regioisomer 3y: 1H NMR (CDCl3 + CCl4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 400 MHz) δ 7.99 (s, 1H), 7.68 (d, J = 8.0 Hz, 2H), 7.47–7.43 (m, 2H), 7.42–7.38 (m, 1H), 4.80 (s, 2H) ppm; 13C NMR (CDCl3 + CCl4 (1
1), 400 MHz) δ 7.99 (s, 1H), 7.68 (d, J = 8.0 Hz, 2H), 7.47–7.43 (m, 2H), 7.42–7.38 (m, 1H), 4.80 (s, 2H) ppm; 13C NMR (CDCl3 + CCl4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 100 MHz) δ 148.8, 137.1, 129.9, 128.9, 120.6, 120.3, 56.2 ppm. 1,5-Regioisomer 3z: 1H NMR (CDCl3 + CCl4 (1
1), 100 MHz) δ 148.8, 137.1, 129.9, 128.9, 120.6, 120.3, 56.2 ppm. 1,5-Regioisomer 3z: 1H NMR (CDCl3 + CCl4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 400 MHz) δ 7.69 (s, 1H), 7.60 (d, J = 7.7 Hz, 2H), 7.47–7.43 (m, 3H), 4.68 (s, 2H) ppm; 13C NMR (CDCl3 + CCl4 (1
1), 400 MHz) δ 7.69 (s, 1H), 7.60 (d, J = 7.7 Hz, 2H), 7.47–7.43 (m, 3H), 4.68 (s, 2H) ppm; 13C NMR (CDCl3 + CCl4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 100 MHz) δ 137.5, 136.3, 129.63, 129.60, 124.7, 120.3, 53.1 ppm.
1), 100 MHz) δ 137.5, 136.3, 129.63, 129.60, 124.7, 120.3, 53.1 ppm.
Acknowledgements
H.S.P.R thanks Special Assistance Program (SAP), University Grants Commission (UGC), and Fund for Improvement of S & T Infrastructure in Universities and Higher Educational Institutions (FIST), Department of Science and Technology (DST) for support. CG thanks to UGC for meritorious fellowship. We thank Central Instrumentation Facility, Pondicherry University and Organic Chemistry Department, Indian Institute of Science, Bangalore for recording spectra.References
- (a) R. Huisgen, G. Szeimies and L. Moebius, Chem. Ber., 1967, 100, 2494–2507 CrossRef CAS; (b) R. Huisgen, Pure Appl. Chem., 1989, 61, 613–628 CrossRef CAS; (c) R. Huisgen and A. Padwa, 1,3-Dipolar Cycloaddition Chemistry, Wiley, New York, 1984, pp. 1–176 Search PubMed; (d) E. Kruiswijk, The Comprehensive e-Book of Named Organic Reactions, Springer, 2nd edn, 2005, p. 869 Search PubMed.
- H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004–2021 CrossRef CAS.
- (a) C. W. Tornøe, C. Christensen and M. Meldal, J. Org. Chem., 2002, 67, 3057–3064 CrossRef PubMed; (b) V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2596–2599 CrossRef CAS.
- (a) C. Schilling, N. Jung and S. Bräse, Organic Azides: Syntheses and Applications, ed. S. Bräse and K. Banert, Wiley, Chichester, UK, 2010, pp. 269–282 Search PubMed; (b) J. E. Hein and V. V. Fokin, Chem. Soc. Rev., 2010, 39, 1302–1315 RSC; (c) P. Wu and V. V. Fokin, Aldrichimica Acta, 2007, 40, 7–17 CAS.
- H. C. Kolb and K. B. Sharpless, Drug Discovery Today, 2003, 8, 1128–1137 CrossRef CAS.
- P. L. Golas, N. V. Tsarevsky, B. S. Sumerlin and K. Matyjaszewski, Macromolecules, 2006, 39, 6451–6457 CrossRef CAS.
- (a) H. Li, F. Cheng, A. M. Duft and A. Adronov, J. Am. Chem. Soc., 2005, 127, 14518–14524 CrossRef CAS PubMed; (b) E. M. Sletten and C. R. Bertozzi, Acc. Chem. Res., 2011, 44, 666–676 CrossRef CAS PubMed; (c) M. D. Best, M. M. Rowland and H. E. Bostic, Acc. Chem. Res., 2011, 44, 686–698 CrossRef CAS PubMed.
- (a) L. Ackermann and H. K. Potukuchi, Org. Biomol. Chem., 2010, 8, 4503–4513 RSC; (b) M. Meldal and C. W. Tornøe, Chem. Rev., 2008, 108, 2952–3015 CrossRef CAS PubMed.
- J. McNulty, K. Keskar and R. Vemula, Chem.–Eur. J., 2011, 17, 14727–14730 CrossRef CAS PubMed.
- D. V. Partyka, L. Gao, T. S. Teets, J. B. Updegraff III, N. Deligonul and T. G. Gray, Organometallics, 2009, 28, 6171–6182 CrossRef CAS.
- Y. Zhou, T. Lecourt and L. Micouin, Angew. Chem., 2010, 122, 2661–2664 (Angew. Chem., Int. Ed., 2010, 49, 2607–2610) CrossRef.
- L. Zhang, X. Chen, P. Xue, H. H. Y. Sun, I. D. Williams, K. B. Sharpless, V. V. Fokin and G. Jia, J. Am. Chem. Soc., 2005, 127, 15998–15999 CrossRef CAS PubMed.
- E. Rasolofonjatovo, S. Theeramunkong, A. Bouriaud, S. Kolodych, M. Chaumontet and F. Taran, Org. Lett., 2013, 15, 4698–4701 CrossRef CAS PubMed.
- (a) W. Carruthers, Some Modern Methods of Organic Synthesis, Cambridge University Press, 1986, pp. 413–414 Search PubMed; (b) Raney Ni was prepared from Ni–Al alloy and stored submerged in EtOH according to the procedure described, in Vogel's Textbook of Practical Organic Chemistry, ed. B. S. Furniss, A. J. Hannaford, P. W. G. Smith and A. R. Tatchell, Longman Scientific & Technical, NY, 1989, 5th edn, pp. 450–451, WARNING: when exposed to air Raney Ni is pyrophoric Search PubMed.
- H. S. P. Rao and C. Guravaiah, Org. Prep. Proced. Int., 2013, 45, 232–240 CrossRef CAS.
- R. Thomas, Practical Guide to ICP-MS: A Tutorial for Beginners, CRC Press, 2013, 3rd edn Search PubMed.
- (a) A. K. Feldman, B. Colasson and V. V. Fokin, Org. Lett., 2004, 6, 3697–3699 Search PubMed; (b) B. Kaboudin, Y. Abedi and T. Yokomatsu, Org. Biomol. Chem., 2012, 10, 4543–4548 RSC.
- R. Saravanakumar, V. Ramkumar and S. Sankararaman, J. Organomet. Chem., 2013, 736, 36–41 CrossRef CAS PubMed.
- S. Chassaing, A. S. S. Sido, A. Alix, M. Kumarraja, P. Pale and J. Sommer, Chem.–Eur. J., 2008, 14, 6713–6721 CrossRef CAS PubMed.
- S. P. Bew, G. D. Hiatt-Gipson, J. A. Lovell and C. Poullain, Org. Lett., 2012, 14, 456–459 CrossRef CAS PubMed.
- (a) V. O. Rodionov, V. V. Fokin and M. G. Finn, Angew. Chem., 2005, 117, 2250–2255 (Angew. Chem., Int. Ed., 2005, 44, 2210–2215) CrossRef; (b) F. Himo, T. Lovell, R. Hilgraf, V. V. Rostovtsev, L. L. Noodleman, K. B. Sharpless and V. V. Fokin, J. Am. Chem. Soc., 2005, 127, 210–226 CrossRef CAS PubMed; (c) L. Zhang, X. Chen, P. Xue, H. H. Y. Sun, I. D. Williams, K. B. Sharpless, V. V. Fokin and G. Jia, J. Am. Chem. Soc., 2005, 127, 15998–15999 CrossRef CAS PubMed.
- (a) S. J. Pastine, S. W. Youn and S. Dalibor, Org. Lett., 2003, 5, 1055–1058 CrossRef CAS PubMed; (b) G. Guilhem, S. Cathy and B. Philippe, J. Med. Chem., 2008, 51, 4374–4376 CrossRef PubMed; (c) M. G. Ana, D. C. Maria, V. J. Serafin and L. Cristobal, Org. Lett., 2002, 4, 383–386 CrossRef PubMed.
- (a) R. F. S. Joseph, J. F. Rebecca, O. R. Valentin and V. F. Valery, Org. Lett., 2010, 12, 4217–4219 CrossRef PubMed; (b) A. Keisuke and M. Seijiro, Org. Lett., 2010, 12, 4988–4991 CrossRef PubMed.
- H. Kang, H. J. Lee, J. C. Park, H. Song and K. H. Park, Top. Catal., 2010, 53, 523–528 CrossRef CAS.
- R. Buckley, S. E. Dann, D. P. Harris, H. Heaney and E. C. Stubbs, Chem. Commun., 2010, 46, 2274–2276 RSC.
- A. Lutz, J. Rajkumar, K. P. Harish, N. K. Petr and B. Lea, Org. Lett., 2010, 12, 2056–2059 CrossRef PubMed.
- B. Y. Lee, S. R. Park, H. B. Jeon and K. S. Kim, Tetrahedron Lett., 2006, 47, 5105–5109 CrossRef CAS PubMed.
- G. A. Jenner, H. P. Longerich, S. E. Jackson and B. J. Fryer, Chem. Geol., 1990, 83, 133–148 CrossRef CAS.
- F. Wang, H. Fu, Y. Jiang and Y. Zhao, Green Chem., 2008, 10, 452–456 RSC.
- (a) A. Marra, A. Vecchi, C. Chiappe, B. Melai and A. Dondoni, J. Org. Chem., 2008, 73, 2458–2461 CrossRef CAS PubMed; (b) A. Dondoni and A. Marra, J. Org. Chem., 2006, 71, 7546–7557 CrossRef CAS PubMed.
- S. Dey and T. Pathak, RSC Adv., 2014, 4, 9275–9278 RSC . It is to be noted that Day and Pathak isolated 1,4-regio isomer although they have wrongly assigned it as 1.5-regioisomer. The NMR data elicited from the spectra matched with the 1,4- rather than 1,5-isomer.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra07057g |
| This journal is © The Royal Society of Chemistry 2014 |